Corrosion Engineering
Principles and Solved Problems
Branko N. Popov
- 792 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
Corrosion Engineering
Principles and Solved Problems
Branko N. Popov
Über dieses Buch
Corrosion Engineering: Principles and Solved Problems covers corrosion engineering through an extensive theoretical description of the principles of corrosion theory, passivity and corrosion prevention strategies and design of corrosion protection systems. The book is updated with results published in papers and reviews in the last twenty years. Solved corrosion case studies, corrosion analysis and solved corrosion problems in the book are presented to help the reader to understand the corrosion fundamental principles from thermodynamics and electrochemical kinetics, the mechanism that triggers the corrosion processes at the metal interface and how to control or inhibit the corrosion rates. The book covers the multidisciplinary nature of corrosion engineering through topics from electrochemistry, thermodynamics, mechanical, bioengineering and civil engineering.
- Addresses the corrosion theory, passivity, material selections and designs
- Covers extensively the corrosion engineering protection strategies
- Contains over 500 solved problems, diagrams, case studies and end of chapter problems
- Could be used as a text in advanced/graduate corrosion courses as well self-study reference for corrosion engineers
Häufig gestellte Fragen
Information
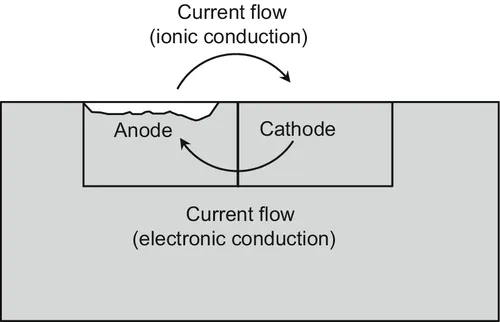
Evaluation of Corrosion
Abstract