1.1Introduction
Carbon dioxide (CO2) is a major greenhouse gas that has triggered global warming and climate change.1–3 Over the past two centuries, its concentration in the atmosphere has rapidly increased, mainly because of human activities such as fossil fuel burning.4–9 However, it is predicted that this trend of increasing atmospheric CO2 concentration with not be altered within the next several decades, because fossil fuels will still be the dominant energy source. Moreover, the energy demand will increase further by 53% by 2030.10 So development of technologies for CO2 capture and storage will become increasingly important.11
Concerns about the increasing concentration of greenhouse gases in the atmosphere have stimulated the study of CO2 capture by using solid adsorbents.12,13 LDHs-derived mixed oxides are considered to be one of the most promising adsorbents for CO2 capture at an intermediate-temperature range (200–400 °C), which require less energy in regeneration and show superior multicycle stability.14,15 In addition, they show fast adsorption/desorption kinetics and good performance in the presence of water,16–18 making them very attractive not only for pre-combustion CO2 capture but also for applications involving CO2 equilibria such as sorption enhanced water gas shift (SEWGS) and sorption enhanced steam reforming (SESR).19–22
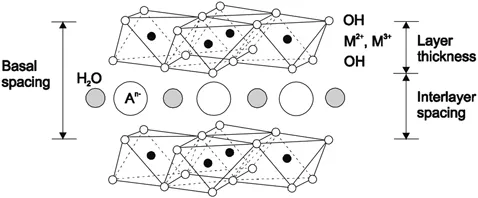
LDHs, also known as hydrotalcite-like compounds or anionic clay, are composed of stacked positively-charged brucite-like layers with interlayer spaces containing charge-compensating anions and water molecules. The metal cations occupy the centers of octahedra whose vertexes contain hydroxide ions. The octahedra are connected by edge sharing to form an infinite sheet.23 It has the composition [M1−x2+Mx3+(OH)2][An−]x/n·ZH2O, where M2+ and M3+ are divalent and trivalent cations, respectively, and An− is the anion that is intercalated in the interlayer for charge compensation.24–28 The general structure of LDHs is shown in Figure 1.1. Because the chemical composition of both the inorganic layers and the interlayer gallery anions can be precisely controlled, LDHs possess highly tunable properties and potentially can be used in a wide range of applications, such as for catalysts, fire retardant additives, polymer–LDH nanocomposites, CO2 capture, luminescent materials, and magnetic materials.29–35 LDHs have been investigated for decades as adsorbents as well.36–38 Owing to their high surface area and abundant basic sites at the surface, LDHs are considered to be favorable for adsorbing acidic CO2.10,39 Fresh LDHs themselves do not possess any basic sites. However, upon thermal treatment, a LDH gradually loses interlayer water; with the temperature increasing, it dehydroxylates and decarbonates to a large extent, leading to the formation of a mixed oxide with a 3D network, with a larger surface area and good stability at high temperature.18,40 Therefore, LDHs are significantly more active for CO2 removal after thermal decomposition, being transformed into basic mixed oxides (LDOs).11
Figure 1.1Schematic structure of LDHs. In this diagram, M2+ and M3+ represent di- and tri-valent cations, which, with the –OH molecules, form the layered structure of the LDHs. The interlayer spacing is occupied with charge-compensating anions (An−) and water molecules. Reproduced from Adsorption, High temperature adsorption of CO2 on various hydrotalcite-like compounds, 14, 2008, 781–789, N. D. Hutson and B. C. Attwood, © Springer Science + Business Media, LLC 2008, with permission of Springer.
Starting from the natural mineral (MgAl–CO3), a lot of work has been done on LDHs-derived CO2 adsorbents, which includes (1) influence of the chemical composition of LDHs, (2) influence of the synthetic conditions and method, (3) LDH-based composites, (4) influence of doping alkali metals, (5) influence of other co-existing gases, (6) adsorption mechanism and kinetics study, and (7) techno-economic assessment etc. Although several recent review papers have provided prolific insights into the progress in this area, most previous reviews have only discussed LDHs-derived CO2 adsorbents as a part of the papers. A detailed review focusing only on LDHs-derived compounds as CO2 adsorbents, addressing their pros and cons, and potential applications in industry is still needed.
In this regard, the objective of this chapter is to review the currently available literature on adsorption of CO2 on LDHs-derived compounds, focusing on the aforementioned seven parts. In addition, based on the overview, the closing section will suggest future research efforts.
1.2Influence of the Chemical Composition of LDHs
As mentioned in the introduction, LDHs are composed of stacked positively-charged brucite-like layers with interlayer spaces containing charge-compensating anions and water molecules.41,42 They are also very open to physicochemical manipulation, numerous combinations of structural cations (Mg2+, Co2+, Cu2+, Ca2+, Fe3+, Al3+ etc.) and interlayer charge-compensating anions (Cl−, NO3−, CO32−, HCO3−, SO42− etc.).43 With a proper understanding of their composition–structure–property relationships, LDHs may conceivably be synthesized and tailored as effective high temperature adsorbents for CO2 separation and capture. Plenty of work has been done to study how substitution of cations and interlayer charge-compensating anions affects the CO2 adsorptive capacity of LDHs.43,44 Yong et al.45 first investigated the effect of Al content in commercial LDHs on their CO2 capture capacity. The adsorption capacities of three kinds of commercial LDHs from CONDEA Chemie Gmbh company (PURAL MG30 (70% Al2O3), MG50 (50% Al2O3), and MG70 (30% Al2O3)) with different aluminium contents were tested at 300 °C and 1 bar. The results showed that the amounts of adsorbed CO2 increased with a ...