
eBook - ePub
Alkaloids
A Treasury of Poisons and Medicines
Shinji Funayama,Geoffrey A. Cordell
This is a test
Condividi libro
- 294 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Alkaloids
A Treasury of Poisons and Medicines
Shinji Funayama,Geoffrey A. Cordell
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Informazioni sul libro
Alkaloids are a large group of structurally complex natural products displaying a wide range of biological activities. The purpose of Alkaloids: A Treasury of Poisons and Medicines is to classify, for the first time, the alkaloids isolated from the natural sources until now. The book classifies all of the alkaloids by their biosynthetic origins. Of interest to the organic chemistry and medicinal chemistry communities involved in drug discovery and development, this book describes many alkaloids isolated from the medicinal plants, including those used in Japanese Kampo medicine.
- Classifies and lists alkaloids from natural sources
- Occurrence and biosynthetic pathways of alkaloids
- Indicates key uses and bioactivity of alkaloids
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Alkaloids è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Alkaloids di Shinji Funayama,Geoffrey A. Cordell in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Naturwissenschaften e Organische Chemie. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
Argomento
NaturwissenschaftenCategoria
Organische ChemieChapter 1
Alkaloids Derived from Phenylalanine and Tyrosine
Abstract
This chapter introduces those alkaloids derived from phenylalanine and tyrosine.
Keywords
Aristolochic acid; Berberine; Chelidonine; Coclaurine; Colchicine; d-tubocurarine; Dopamine; Emetine; Galanthamine; L-DOPA; Lycorine; Morphine; Phenylethylamine; Thyroxine
The thousands of alkaloids derived from phenylalanine and tyrosine possess a wide range of important biological activities, and several of them are pharmaceutical agents, present in various traditional medicines in various systems, or serve as biological tools. In this chapter, those alkaloids are discussed, from the simplest alkaloids to those that represent more complex chemical structures. The coclaurine-type alkaloids are one of the simplest of these alkaloids, and are described in Section 1.4. Among them, reticuline, in its antipodal forms, is an important biosynthetic precursor of various alkaloids, including such alkaloids as berberine and morphine. Some of the alkaloids of this type are derived through highly complicated, and incompletely understood, biosynthetic pathways. For example, it is very difficult to elucidate the original amino acid derivations of colchicine (Section 1.11) and lycorine (Section 1.13) from a superficial examination of their chemical structures.
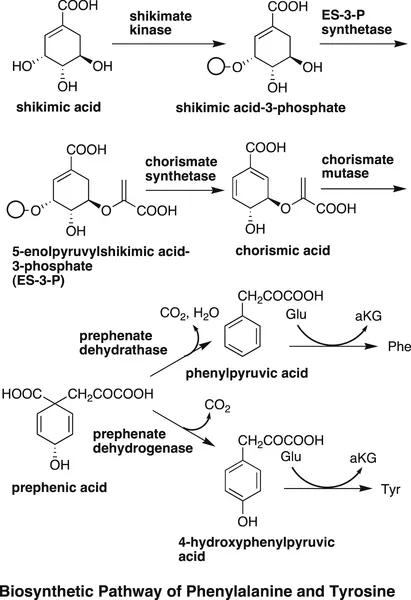
Both phenylalanine and tyrosine are derived from chorismic acid, which is itself derived from shikimic acid-3-phosphate through the shikimic acid pathway. In this sequence, chorismic acid is first transformed into prephenic acid by chorismate mutase. If prephenic acid is converted into phenylpyruvic acid by the action of prephenate dehydratase and a transaminase, phenylalanine is formed. On the other hand, if it is transformed into 4-hydroxyphenylpyruvic acid by the action of prephenate dehydrogenase, followed by a transaminase reaction, then tyrosine is formed.
1.1
Phenylethylamines (Phenethylamines)
Peyote (Lophophora williamsii, syn. Anhalonium williamisii) is a cactus and member of the family Cactaceae, and grows wild in the deserts of Mexico and the southern United States [1]. The cactus is also cultivated in Japan as a decorative plant and known as “Ubatama.”
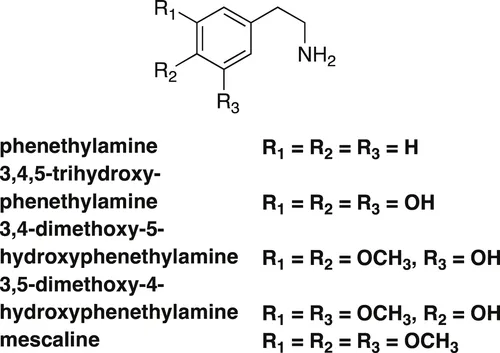
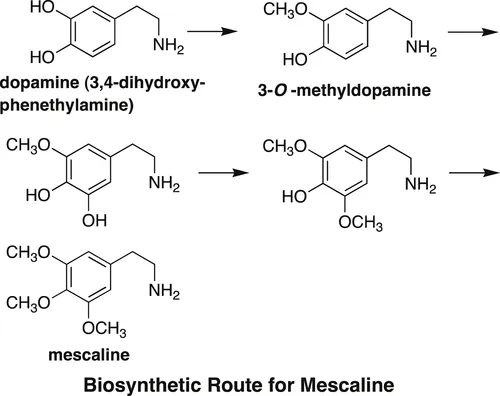
This cactus is an important source of the so-called phenylethylamine (phenethylamine) alkaloids with a C6C2N skeleton. The main component of the alkaloid mixture is mescaline. The name mescaline is derived from the name of the cactus, which is also known as “mescal buttons.” Mescaline is known to possess hallucinatory effects and a number of undesirable side effects. With respect to the biosynthesis of mescaline, it was shown that L-tyrosine is oxidized to give L-DOPA (L-3,4-dihydroxyphenylalanine), which is transformed to the biosynthetic precursor dopamine (3,4-dihydroxyphenethylamine) [2], which is selectively O-methylated to afford 3-O-methyldopamine, a key biosynthetic precursor of the alkaloid. The direct biosynthetic precursor of mescaline was determined to be 3,5-dimethoxy-4-hydroxyphenethylamine, because 3,4,5-trihydroxy phenethylamine and 3,4-dimethoxy-5-hydroxyphenethylamine were not incorporated into the biosynthetic pathway to mescaline [2].
Hordenine and N-methyltyramine are isolates from the young roots of Hordeum vulgare var. hexastichon (Poaceae), and are simple phenylethylamine-type alkaloids. The biosynthetic precursor of these alkaloids is considered to be tyramine, derived from tyrosine. dl-[2-14C]-Tyrosine was fed to H. vulgare var. hexastichon 4 days after germination, and hordenine and N-methyltyramine were isolated after 11 days from the roots. Both alkaloids possessed 14C label at the α-carbon. It was also found that dl-[2-14C]-tyrosine was more effectively incorporated into N-methyltyramine than into hordenine, and no tyramine was detected in the extract. So, the incorporated tyrosine was converted into tyramine and methylated immediately to give N-methyltyramine. Subsequent steps form hordenine by the methylation of N-methyltyramine [3].
On the other hand, it was clarified by the incorporation of labeled methionine that the methyl groups incorporated during the biosynthesis of N-methyltyramine and hordenine were derived from methionine. The yield of N-methyltyramine in these experiments is less than half that of hordenine; however, the incorporation of 14C into N-methyltyramine is 1.5 times that of hordenine. This indicates that N-methyltyramine is not formed by the demethylation of hordenine, but th...