
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
An updated fourth edition of the text that provides an understanding of chemical transformations and the formation of structures at surfaces
The revised and enhanced fourth edition of Surface Science covers all the essential techniques and phenomena that are relevant to the field. The text elucidates the structural, dynamical, thermodynamic and kinetic principles concentrating on gas/solid and liquid/solid interfaces. These principles allow for an understanding of how and why chemical transformations occur at surfaces. The author (a noted expert on in the field) combines the required chemistry, physics and mathematics to create a text that is accessible and comprehensive.
The fourth edition incorporates new end-of-chapter exercises, the solutions to which are available on-line to demonstrate how problem solving that is relevant to surface science should be performed. Each chapter begins with simple principles and builds to more advanced ones. The advanced topics provide material beyond the introductory level and highlight some frontier areas of study. This updated new edition:
- Contains an expanded treatment of STM and AFM as well as super-resolution microscopy
- Reviews advances in the theoretical basis of catalysis and the use of activity descriptors for rational catalyst design
- Extends the discussion of two-dimensional solids to reflect remarkable advances in their growth and characterization
- Delves deeper into the surface science of electrochemistry and charge transfer reactions
- Updates the "Frontiers and Challenges" sections at the end of each chapter as well as the list of references
Written for students, researchers and professionals, the fourth edition of Surface Science offers a revitalized text that contains the tools and a set of principles for understanding the field.
Instructor support material, solutions and PPTs of figures, are available at http://booksupport.wiley.com
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Surface and Adsorbate Structure
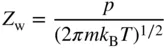
1.1 Clean surface structure
1.1.1 Ideal flat surfaces
Table of contents
- Cover
- Table of Contents
- Preface
- Supplementary Material
- Introduction
- 1 Surface and Adsorbate Structure
- 2 Experimental Probes and Techniques
- 3 Chemisorption, Physisorption, and Dynamics
- 4 Thermodynamics and Kinetics of Adsorption and Desorption
- 5 Liquid Interfaces
- 6 Heterogeneous Catalysis
- 7 Growth and Epitaxy
- 8 Laser and Non‐thermal Chemistry: Photon and Electron Stimulated Chemistry and Atom Manipulation
- Appendix A:Appendix AAbbreviations and Prefixes
- Appendix B: Appendix BSymbolsSymbols
- Appendix C:Appendix CUseful Mathematical ExpressionsUseful Mathematical Expressions
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app