![]()
In its quest to certify endodontists with the highest levels of knowledge, the American Board of Endodontics (ABE) examination process focuses on the practice of evidence-based dentistry, wherein the provider makes treatment decisions based on a comprehensive and constantly evolving evaluation of the bodies of research and literature in their field. Practitioners must sift through the available resources with a discerning eye.
In each section of the ABE examination, candidates must demonstrate their ability to justify their decisions and recommendations based on the highest-quality evidence available. Research published in peer-reviewed journals is considered to be unbiased and therefore most useful. Although textbooks and lectures are effective means of disseminating information, quality versions of these resources will refer back to primary resources in peer-reviewed journals. Consequently, it is imperative that providers familiarize themselves with the primary references cited in all examples. This chapter will cover study design, measures of statistical significance and validity, and epidemiology. For a more in-depth review of research design and biostatistics, please refer to Hulley et al’s Designing Clinical Research or Glaser’s High-Yield Biostatistics.
Study Design
Beyond citing peer-reviewed journals as the ideal reference source, certain study designs are generally considered more scientifically sound. The Oxford Centre for Evidence-Based Medicine (OCEBM) outlines a hierarchy of levels of evidence by study design, illustrated in Fig 1-1.
Fig 1-1 OCEBM hierarchy of levels of evidence by study design.
Systematic reviews, including meta-analyses, are considered the highest level of evidence, and their quality improves based on the compiled levels of evidence of the studies reviewed. Systematic reviews involve a comprehensive search and review of all of the literature on a topic, and a meta-analysis delves deeper by doing statistical analyses to make direct comparisons between studies. Depending on the variability of the statistics reported in the literature available on a topic, a meta-analysis may not be achievable. Systematic reviews and meta-analyses are limited by the quality of the studies included, and the following discussion of levels of evidence should be taken into account in evaluation of the quality of literature reviews.
Looking next to clinical research studies, randomized controlled trials are considered the highest level of evidence (OCEBM). Randomized controlled trials involve a planned intervention on a diseased population with matched controls. These studies are both resource- and time-intensive and are consequently difficult to perform. Futhermore, ethical concerns often arise in the discussion of this study type. Prior knowledge of superior intervention outcomes cannot be denied from a diseased population, and it is considered unethical to study certain populations, such as children or the disabled.
Cohort studies are considered next best among the levels of evidence hierarchy (OCEBM). Cohort studies are prospective and longitudinal and measure for the incidence of new cases of a disease while assessing risk or protective factors. These types of studies can be resource intensive and are not practical for rare outcomes.
Case-control studies follow cohort studies in the OCEBM hierarchy. This type of study compares past risk factors and exposures of cases with disease and controls without disease in a retrospective fashion. These studies are often less expensive to perform and less time intensive and can be useful to study rare outcomes. They are considered lower quality due to recall bias, difficulties with misdiagnosis, and assignment of controls.
Publications of case series or case reports represent the lowest level of evidence for observational studies (OCEBM). They involve a simple presentation of an outcome without provision of a control.
Lastly, expert opinions offer the lowest level of evidence. Their utility is limited in the justification of evidence-based diagnosis and treatment. Rather, they serve to introduce innovation and new techniques as clinical empiricism is oftentimes the starting point for further higher-level research.
Statistics
Although a comprehensive review of biostatistics will not be addressed in this textbook, a review of the more commonly encountered concepts in biostatistics, particularly those encountered in later parts of this text, will be presented. Readers are encouraged to seek out further resources, particularly if questions arise during the reading of primary references.
Measures of statistical significance
The ultimate goal of research is to test a hypothesis. Although absolute statements regarding proof or disproof of a hypothesis cannot be made based on limited populations and study parameters, researchers look to determine the likelihood that results support the hypothesis. Similarly, determination of cause and effect is extremely difficult to prove, requiring large-scale randomized controlled trials with longitudinal follow-ups. Most studies fall short of determining causation but can identify associations or relationships between two factors. It is important in quoting literature to never overstate results.
One way researchers can increase the odds of obtaining statistically significant results is to ensure that the sample population under study is both large and diverse enough to demonstrate outcomes. Although successful completion of the ABE examination does not require an intimate understanding of the methods researchers use to determine the adequacy of sample sizes, familiarity with the concept of power to rule out errors in hypothesis testing is imperative. Well-designed research studies involve power calculations to assure adequate sample sizes, and in critical review of literature articles, one should note if appropriate power calculations were made to justify the use of a particular sample size.
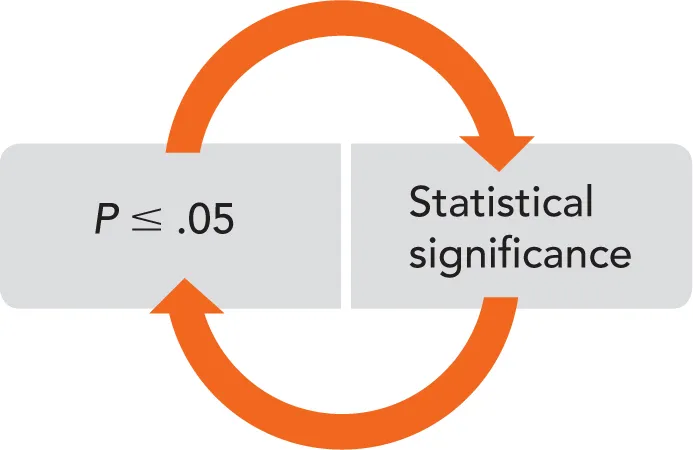
With samples selected and the experiment performed, results must be analyzed to determine their statistical relevance. The most common measure of statistical significance encountered in the endodontic literature is the P value. The P value refers to the likelihood of the outcome having occurred by chance. A P value less than or equal to .05 generally indicates statistical significance (Fig 1-2). In other words, with a P value of less than .05, the probability that the study results were obtained by chance is less than 5%. For example, in a retrospective case-control study performed by Spili et al investigating the outcomes of teeth with and without fractured nickel-titanium instruments, 91.8% success was found in cases with retained fractured instruments compared with 94.5% success in controls. Statistical analysis using the Fisher exact test, a tool used to determine deviation from a null hypothesis, resulted in a P value of .49. This corresponds to a 49% chance that the difference in healing rates was due to chance. As the authors set the significance value at P = .05, the difference in healing rates obtained from the study was deemed statistically insignificant.
Fig 1-2 The relationship between P value and statistical significance. The P value describes the probability that results occurred by chance.
Measures of validity
When new testing modalities are compared to the current standard, the validity or accuracy of the new approach must be verified. Sensitivity, specificity, and predictive values provide the means by which validity can be confirmed (Fig 1-3). These values are often encountered in descriptions of pulp sensitivity tests. Jespersen et al’s study on the validity of cold sensitivity testing using Endo Ice [Hygenic] provides an excellent example in the discussion of validity measures.
Fig 1-3 The validity measures often encountered in the endodontic literature.
Understanding validity measures requires familiarity with the concepts of both true positive and negative results and false positive and negative results (Table 1-1). True positive or negative results correctly identify individuals as healthy or diseased. False positive or negative results incorrectly identify the individual’s disease status.
Table 1-1 The possible outcomes of a test
Test result | Disease present | Disease absent |
Positive | True positive | False positive |
Negative | False negative | True negative |
Sensitivity is defined as the ability of a test to detect diseased individuals. It is calculated by comparing the number of true positives detected by the test with the total number of diseased subjects, including the true positives plus false negatives. In Jespersen’s study, the sensitivity was 0.92 for cold testing. In other words, 92% of teeth with pulpal necrosis were correctly identified.
Specificity is defined as the ability of a test to correctly identify a healthy individual. It is calculated by comparing the number of true negatives detected by the test with the total number of nondiseased subjects, including the true negatives and false positives. In Jespersen’s study, the specificity was 0.90 for cold testing. In other words, the cold test correctly identified vital teeth 90% of the time.
Predictive values describe the likelihood of the test to correctly identify health or disease. ...