
eBook - ePub
Handbook of Biochemistry
Section C Lipids Carbohydrates & Steroids, Volume l
- 586 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This first volume contains data on amino acids which consists of the coefficients of solubility in water, heat capacities, entropies of formation, and heats of combustion. Specific gravity liquids, sucrose solution, CsCI solution isokinetic glycerol and sucrose gradients for density gradient centrifugation and the temperature dependence for select compounds are included.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Table 1
NATURAL ALDITOLS, INOSITOLS, INOSOSES, AND AMINO ALDITOS AND INOSAMINES
NATURAL ALDITOLS, INOSITOLS, INOSOSES, AND AMINO ALDITOS AND INOSAMINES









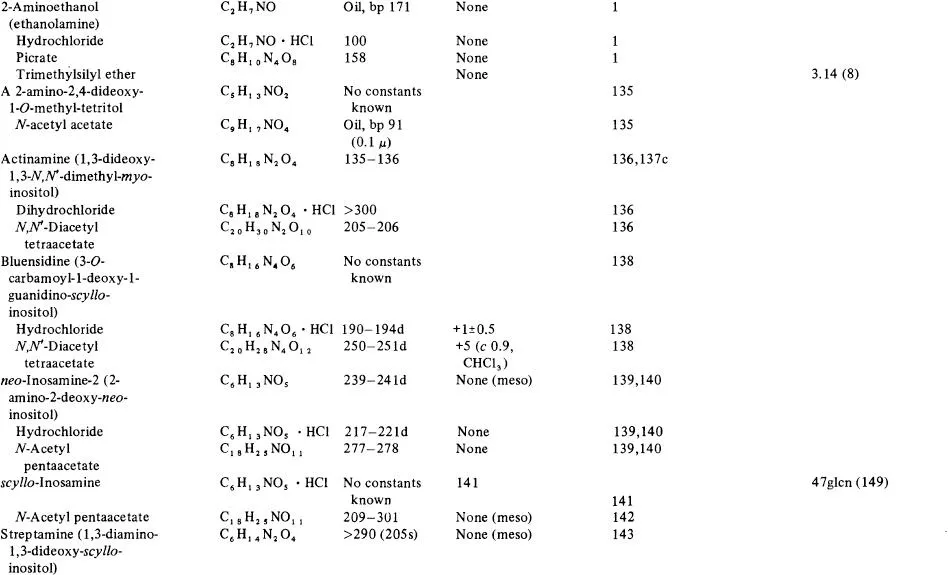
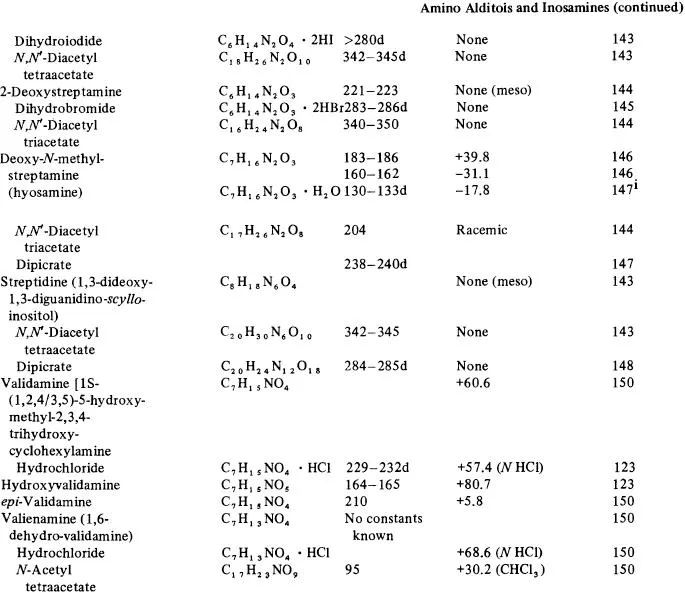
a In order of increasing carbon chain length in the parent compounds grouped in the classes-alditols, inositols, inososes, amino alditols, and inosamines.
b [α]D for 1–5 g solute, c, per 100 ml aqueous solution at 20–25°C, unless otherwise given.
c References for melting point and specific rotation data. Letter indicates the reference also has chromatographic data according to: c = column, e = electrophoresis, g = gas, p = paper, and t = thin-layer.
d R value times 100, given relative to that of the compound indicated by abbreviation: f = solvent front, ala = alanine, ara = arabinose, asa = ascorbic acid, Cl = chloride ion, eic = eicosane, gain = galactono-1,4-lactone, galU = galacturonic acid, glc = glucose, glcN = glucosamine, glcU = glucuronic acid, gNAc = 7V-acetyl-glucosamine, gNUA = glucosaminuronic acid, kdh= 3-deoxy-erythro-hexulosonic acid, kdo= 3-deoxy-mtfw«o-octulosonicacid, kgu = 2-keto-gulonic acid, mal = malonic acid, manU = mannuronic acid, myo = myo-inositol, myot = myo-inositol trimethylsilyl ether, MU = methylene standard hydrocarbon units, nana = W-acetyl-neuraminic acid, pa = picric acid, rha = rhamnose, tmg = 2,3,4,6-tetra-O-methyl glucose, and xyll = xylitol pentamethylether. Under gas chromatography (column Glc or G) numbers without code indications signify retention time in minutes. The conditions of the chromatography are correlated with the reference given in parentheses and are found in Table 5.
e said to exit as a phosphate ester also.10
f Data given are for the enanthiomorphic isomer.
g The author name as 3-dehydroquinic acid, but it is actually 5-dehydroquinic.
h The early given name, 1-quercitol, of this compound does not make it the enanthiomorph of d-quercitol; other isomeric relations are involved.
i This compound is isomeric with the previous one in regard to the N-methy)group position.
Compiled by George G. Maher.
REFERENCES
1. Pollock and Stevens, Dictionary of Organic Compounds, Oxford University Press, New York, 1965.
2. Frahn and Mills, Aust. J. Chem., 12, 65 (1959).
3. Dooms, Declerck, and Verachtert, J. Chromatogr., 42, 349 (1969).
4. Bourne, Lees, and Weigel, J. Chromatogr., 11, 253 (1963).
5. de Simone and Vicedomini, J. Chromatogr., 37, 538 (1968).
6. Sawardeker, Sloneker, and Jeanes, Anal. Chem., 37, 1602 (1965).
7. Whyte, J. Chromatogr., 87, 163 (1973).
8. Roberts, Johnston, and Fuhr, Anal. Biochem., 10, 282 (1965).
9. Dutton and Unrau, Can. J. Chem., 43, 924, 1738 (1965).
10. Lindberg, Ark. Kemi. Mineral Geol., 23, A 2 (1946–1947).
11. Weatherall, J. Chromatogr., 26, 251 (1967)...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- NOMENCLATURE
- CARBOHYDRATES
- LIPIDS
- STEROIDS
- INDEX
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Handbook of Biochemistry by Gerald D Fasman in PDF and/or ePUB format, as well as other popular books in Medicine & Biochemistry in Medicine. We have over one million books available in our catalogue for you to explore.