
- 448 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Infrared Spectroscopy for Food Quality Analysis and Control
About This Book
Written by an international panel of professional and academic peers, the book provides the engineer and technologist working in research, development and operations in the food industry with critical and readily accessible information on the art and science of infrared spectroscopy technology. The book should also serve as an essential reference source to undergraduate and postgraduate students and researchers in universities and research institutions.Infrared (IR) Spectroscopy deals with the infrared part of the electromagnetic spectrum. It measure the absorption of different IR frequencies by a sample positioned in the path of an IR beam. Currently, infrared spectroscopy is one of the most common spectroscopic techniques used in the food industry. With the rapid development in infrared spectroscopic instrumentation software and hardware, the application of this technique has expanded into many areas of food research. It has become a powerful, fast, and non-destructive tool for food quality analysis and control.Infrared Spectroscopy for Food Quality Analysis and Control reflects this rapid technology development. The book is divided into two parts. Part I addresses principles and instruments, including theory, data treatment techniques, and infrared spectroscopy instruments. Part II covers the application of IRS in quality analysis and control for various foods including meat and meat products, fish and related products, and others.
- Explores this rapidly developing, powerful and fast non-destructive tool for food quality analysis and control
- Presented in two Parts -- Principles and Instruments, including theory, data treatment techniques, and instruments, and Application in Quality Analysis and Control for various foods making it valuable for understanding and application
- Fills a need for a comprehensive resource on this area that includes coverage of NIR and MVA
Frequently asked questions
Information
Principles of Infrared Spectroscopy
Publisher Summary
Introduction
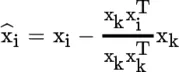
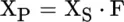
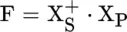
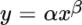
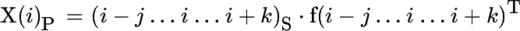
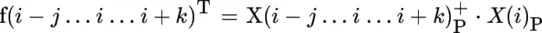
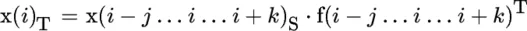
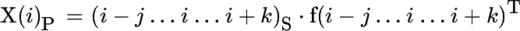

Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- About the Editor
- Contributors
- Preface
- Part I: Fundamentals And Instruments
- Part II: Applications
- Index