![]()
SECTION III
GAS WELDING AND CUTTING
General
Safety precautions
Equipment
Acetylene
Oxygen
Regulators
Welding torches
Setting up equipment
Oxyacetylene flame
Adjusting the flame
Joint design
Welding methods
Expansion and contraction
Welding iron and steel
Bronze welding or brazing
Welding aluminum
Welding copper
Welding brass and bronze
Oxyacetylene cutting
Backfire and flashback
Special precautions
13. General.—Fusion welding is the process of joining metals by melting or fusing together adjacent surfaces or edges without applying pressure. The heat for fusing the metal may be provided either by gases or electricity. Fusion welding is an important function of every military automotive maintenance shop. By use of the oxyacetylene process, iron and steel can be severed, shaped, or joined, and other metals can be joined. Permanent repairs can usually be made by welding, even in the field, avoiding the necessity for temporary or “stop-gap” repairs.
a. The gases commonly used for welding or cutting are oxygen and and acetylene, although oxygen with some other fuel gas is sometimes used. This section describes the oxyacetylene process. Electric welding will be described in section IV. Almost the same results can be accomplished by either process. The oxyacetylene outfit is somewhat more portable than the electric and is usually available even in mobile shops. The oxyacetylene process cuts steel and iron very efficiently.
b. An important use of the oxyacetylene process, especially in the small shop or in the field, is for heating. When forges and other blacksmithing equipment are not available, simple bending and forming operations can be performed easily by using the welding torch to heat the metal to forging temperature, or for annealing, hardening, and tempering operations, if necessary.
14. Safety precautions.—Acetylene is an inflammable gas that is explosive when mixed with air. Oxygen is a chemically active gas. Both are compressed into cylinders under high pressures for industrial uses, and both are extremely dangerous unless the safety precautions mentioned throughout this section and in the manufacturers’ literature are strictly observed. One of the most important of these is never to oil regulators, torches, or any part of welding or cutting apparatus and never let oil or grease come in contact with or be near oxygen. Oil or grease coming in contact with pure oxygen under pressure will ignite violently or explode.
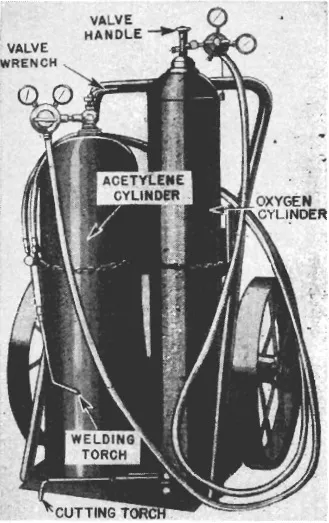
15. Equipment.—In its simplest form an oxyacetylene welding and cutting outfit consists of a cylinder of oxygen, a cylinder of acetylene, two regulators, two lengths of hose with fittings, and a welding torch supplemented by a cutting attachment or a separate cutting torch. With this equipment all commercial metals can be welded; steel, wrought iron or cast iron can be cut; and local metal heating operations can be done effectively. Mounted on a hand truck as shown in figure 33, such an outfit is portable and can be taken wherever the work is located. In addition, goggles are required to protect the welder’s eyes, gloves to protect his hands, and a friction lighter to light the torch without danger of burning them. Wrenches are needed for cylinders, regulators, and torches. Welding rod or flux, or both, are also usually required. Interchangeable tips or heads of different sizes must be provided for the torches if the same handles are to be used for a variety of operations.
16. Acetylene.—a. Acetylene is a fuel gas having a distinctive odor, composed of carbon and hydrogen, its chemical formula being C2H2. It is manufactured by the action of water on calcium carbide, a crushed, rock-like substance produced in an electric furnace from coke and limestone. The gas is contained in steel cylinders under a pressure of about 225 pounds per square inch when the cylinder is full. An acetylene cylinder is not hollow; it is filled with a porous material saturated with acetone, a liquid chemical which absorbs many times its own volume of acetylene. The acetylene is dissolved in the acetone under pressure in the same way that carbon dioxide gas (CO2) is dissolved under pressure in a bottle of soda water. This treatment makes the acetylene safe, even under the high 225-pound pressure, and allows enough acetylene to be stored in a cylinder for several days’ use under average conditions. The usual capacity of an acetylene cylinder is about 300 cubic feet. The cylinder itself is a strong steel container provided with a valve for attaching the regulator and drawing off the acetylene. Acetylene, once it has left the cylinder, is called “free.” In this state it is liable to explode spontaneously when used at gage pressure over 15 pounds per square inch. A safety fuse plug which melts and releases the gas from the container is provided in case of fire. A cylinder of acetylene of 300 cubic feet capacity weighs 232.5 pounds full and 214 pounds empty. Smaller cylinders containing approximately 100 cubic feet are available. Empty cylinders must be returned to the manufacturer for recharging.
FIGURE 33.—Portable oxyacetylene welding outfit.
b. For safety’s sake, remember that acetylene will burn and will form explosive mixtures with air. Handle acetylene cylinders carefully and store them in a well-ventilated, dry place away from combustible materials, stoves, radiators, or furnaces. Keep the valve end up. Never tamper with fuse plugs.
17. Oxygen.—a. Oxygen is a colorless, odorless, and tasteless gas. It is necessary for the combustion of most substances and combines chemically with them when they burn. Oxygen itself is not inflammable but is said to support combustion. For ordinary fires, such as forge and furnace fires, the oxygen in the air supports combustion of the fuel. Except for a very small percentage of rare gases, such as argon, neon, and helium, the atmosphere is composed of about one-fifth oxygen and four-fifths nitrogen. Nitrogen does not aid in combustion but merely cools off the fire. It can be seen, therefore, that the pure oxygen is a much more active agent than air in supporting combustion.
b. The bulk of the oxygen used in industry today is obtained from the atmosphere by the liquid air process; a small amount is made from water by the electrolytic process. Air is liquefied by a process of compression, cooling, and expansion, and oxygen is separated from the resulting extremely cold liquid by fractional distillation, in which advantage is taken of the fact that liquid nitrogen boils at a lower temperature than liquid oxygen. The distilling apparatus delivers practically pure oxygen to a storage holder, from which it is compressed into steel cylinders for distribution.
c. Oxygen is produced by the electrolytic process, as follows: Water is a chemical compound of hydrogen and oxygen (H2O), each of which is a gas in its uncombined state. Under suitable conditions, an electric current passed through water decomposes it into its two elements. Bubbles of oxygen rise from the positive electrical terminal or electrode and hydrogen bubbles from the negative electrode. Each gas is led to a storage holder and then compressed in cylinders.
d. Oxygen cylinders are very strong, seamless, drawn-steel containers into which the oxygen is compressed to the extremely high pressure of 2,000 pounds per square inch. This pressure is measured at 70° F. and becomes somewhat greater when the cylinder is at a higher temperature and somewhat less when it is at a lower temperature. The pressure decreases as the oxygen is drawn off and the cylinder emptied. Unlike acetylene cylinders, oxygen cylinders are hollow. They are provided with specially designed valves to resist the high pressure. Every oxygen cylinder valve has some kind of a safety device to blow off the oxygen in case the cylinder is directly exposed to fire. The usual oxygen cylinder contains 220 cubic feet of oxygen and is 56 inches in height (including valve) and 9 inches in diameter. It weighs about 148 pounds full and 130 pounds empty. Smaller cylinders containing 110 cubic feet are available. The cylinder must be returned to the manufacturer when empty.
e. Oxygen is not an inflammable gas but causes other burning materials to burn more violently when they are exposed to it. It will cause oily or greasy substances to burst into flame with explosive violence without any other ignition. Always remember this when using it. Never confuse oxygen with compressed air and never use it to supply head pressure on a tank. It would be fatal to put pressure on the tank of a kerosene preheating torch, for instance. Never use oxygen for pneumatic tools, to start internal combustion engines, to blow out pipe, or to “dust” clothes. Oxygen cylinders are strong enough to withstand ordinary handling, but they should not be dropped off platforms, knocked about, or placed where heavy articles may drop on them. Do not store them in hot places or where oil may drop on them from overhead bearings or machines.
FIGURE 34.—Oxygen and acetylene regulators.
18. Regulators.—a. General.—The function of a regulator (fig. 34) is to reduce the high pressure of the gas in the cylinder to a low constant working pressure at the torch. For example, a cylinder contains oxygen at a pressure of 2,000 pounds per square inch, and a pressure of only 10 pounds per square inch is wanted at the torch to do some welding. The regulator must reduce the cylinder pressure from 2,000 to 10 pounds per square inch and must maintain this working pressure as the cylinder pressure decreases. Therefore, a regulator must have a sensitive regulating mechanism in addition to a reducing valve.
(1) A regulator for either an acetylene or an oxygen container has a union nipple for attaching it to the cylinder and an outlet connection for the hose leading to the torch. There are two gages on the body of the regulator: one shows the pressure in the cylinder, and the other the working pressure being supplied to the torch. The working pressure is adjusted by a hand screw. When this screw is turned to the left (counterclockwise) until it turns freely, the valve inside the regulator is closed and no gas can pass to the torch. Turning the handle to the right (clockwise) opens the valve, and gas passes to the torch at the pressure shown on the working pressure gage. The more the hand screw is turned to the right, the higher the pressure shown on the working pressure gage. Typical regulators are shown in figure 34.
(2) Before opening the valve on a cylinder to which a regulator is attached, the pressure-adjusting screw must be fully released by turning it to the left (counterclockwise) until it turns freely. Otherwise, when the cylinder valve is opened, th...