1Human pluripotent stem-cell-derived vascular cells: in vitro model for angiogenesis and drug discovery
Abstract: Angiogenesis is the process of formation of new blood vessels in development, health and diseased states. It is an intricate process involving a multitude of overlapping phases that encompass cell-cell and cell-matrix interactions between the vascular cells (in particular, endothelial cells and vascular smooth muscle cells/pericytes) and the surrounding extracellular matrix. Assays to investigate angiogenesis are used in the screening of novel proangiogenic and antiangiogenic drug candidates that have potential in the treatment of ischemic diseases, cancers, a range of vascular disorders and for therapeutic vascularization in regenerative medicine. Most assays rely on in vitro models of angiogenesis using monolayer cultures of primary and immortalized ECs and in vitro animal models. Pluripotent stem cells (PSCs) like human embryonic stem cells (ESCs) due to their ability to self-renew, differentiate to any cell type in the body, and recapitulate the embryological, physiological, and pathological states of angiogenesis, provide a promising in vitro cellular platform for development of novel proangiogenic and antiangiogenic drug candidates. This chapter highlights the benefits of using human PSC-derived vascular cells for drug discovery, the methods developed in our laboratory for differentiation of human ESCs to vascular cells and various co-culture models for drug screening studies. Further, we will discuss some of the obstacles and challenges that need to be addressed for stem-cell-based approaches to be used as an effective tool in the pursuit to develop clinically successful drug compounds.
1.1Introduction
With the tremendous amount of investment and growth in automation and highthroughput screening, increasingly large number of compound libraries are synthesized and screened for angiogenesis promoters and inhibitors. However, the discovery of drugs targeting angiogenesis is hampered by the wide gap between lead compound validation and its clinical success. One of the primary reasons for this inefficiency in translating lead compounds into the clinic is the unpredictability of the currently used in vitro angiogenesis assays and the complexity of in vivo microenvironment. The vascular system is composed of a complex and heterogeneous three-dimensional (3D) organization of endothelial cells (ECs) and mural cells (that include pericytes, vascular smooth muscle cells (vSMCs), and fibroblasts) within the surrounding extracellular matrix (ECM). To compound this complexity, both ECs and mural cells are heterogeneous populations with differences in gene expression and functional phenotype among different vessels (arteries, veins, arterioles, venules, capillaries and lymphatics) and among different tissues and organs [1–5]. On the contrary, most highthroughput screening assays utilize monolayer cultures of primary or immortalized ECs without the 3D microenvironment and their interaction with surrounding mural cells and ECM, a highly reductionist approach wherein the crucial elements of drugbiology interaction are lost [6]. Furthermore, the lead compounds are validated and optimized in similar simplified in vitro systems and animal models. Based on the regulatory requirements, lead compounds are validated on at least two animal models (one rodent and one non-rodent species). Numerous data have confirmed the limited predictive value of animal models (especially rodent assays) in the human context due to fundamental differences in the physiology between humans and animals [7, 8].
These shortcomings eventually lead to clinical failures and dramatic increases in the costs of the drug discovery process that are recognized by the pharmaceutical industry, yet few solutions are currently available. This demand for better in vitro angiogenesis models has triggered researchers within academia and pharmaceutical industry to develop physiologically more relevant in vitro human models for screening and validation. From this perspective, this book chapter highlights the promise of human pluripotent stem cells (PSCs) and their vascular progenies for developing angiogenesis assays and their use in drug screening. Further, we will discuss some of the obstacles and challenges that need to be addressed for stem-cell-based approaches for use as an effective tool in pursuit to develop clinically successful drug compounds.
1.2Formation of blood vessels
Neoangiogenesis is the formation of new blood vessels during embryonic development and postnatal life that can occur through two distinct processes, namely vasculogenesis and angiogenesis. Vasculogenesis is the process of formation of primitive plexus of vessels de novo from differentiation of vascular progenitors [9, 10]. Vasculogenesis predominantly occurs during the early stages of embryonic development. In the embryo, vascular progenitors derived from lateral plate mesoderm give rise to ECs that form the primitive blood vessels. Mounting evidence from the published data suggests that vasculogenesis occurs in the postnatal life as well through the endothelial progenitors in the bone marrow and circulating angiogenic cells.
Angiogenesis, however, is the formation of new blood vessels from a pre-existing vascular network. This process is involved in the development of vasculature in the embryo, growing tissues, tissue repair, uterine endometrium and in diseased states including cancer [11]. Angiogenesis is a complex morphogenetic process that implicates both cellular and extracellular components and includes the following events:
- Activation of resting ECs of the target (preexisting) vessel to a migratory EC phenotype.
- Degradation of ECM surrounding the target blood vessel.
- Migration of activated ECs through the basement membrane into the surrounding stromal tissues.
- Proliferation of the activated ECs to form solid sprouts or vascular cords directed towards the angiogenic source.
- Migration and proliferation of mural cells (pericytes and/or vascular smooth muscle cells) along the perimeter of the vascular cords. Mural cells are contractile and supportive cells that are associated with all vascular channels and play a role in vascular maturation, vascular remodeling and maintaining the vessel tone [12]. These mural cells are mostly found in the larger vessels (arteries and veins) and “are known as vSMCs, whereas the ones present in association with the smaller channels (arterioles, capillaries, venules) are referred to as pericytes [13].
- Lumenization of the vascular cords resulting in patent blood vessels.
- Remodeling and maturation of the newly formed blood vessels through the recruitment of mural cells. Formation of mature and functional microvascular network relies on the interaction between ECs and mural cells [14, 15]. During early embryogenesis and throughout the postnatal life, newly formed endothelial vessels recruit mural cells resulting in a complex network of arteries, arterioles, capillaries, venules and veins [9, 10]. Without the mural cells, neo-endothelial structures undergo regression thus suggeting the crucial role of mural cells in providing structural and functional support to the nascent endothelial vessels [11, 15].
1.3Current models to assess angiogenesis
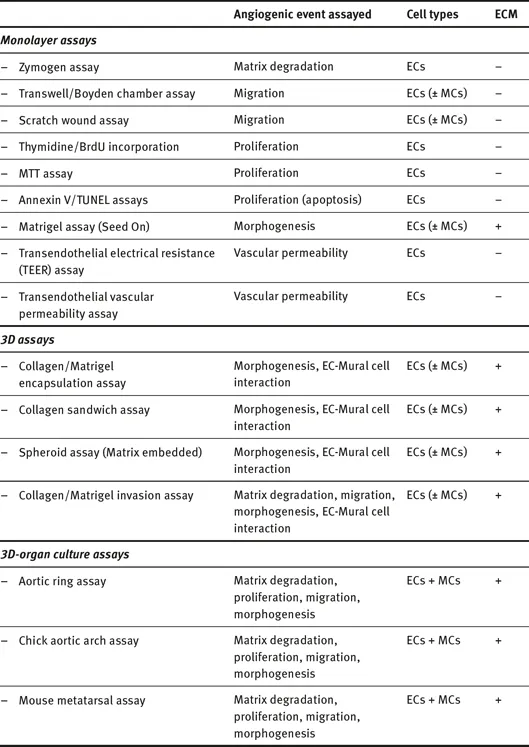
Insufficient vascularization contributes to the pathogenesis of ischemic disorders such as myocardial infarction, peripheral vascular disease and stroke. However, abnormal or excessive vascularization can contribute to the pathogenesis of cancer, diabetic retinopathy, macular degeneration, psoriasis, vascular malformations and other diseases [11]. Therapeutic strategies using proangiogenic and antiangiogenic agents that enhance or decrease the growth of blood vessels, respectively, could be used as therapeutic targets to treat these disorders. Various in vitro assays are currently used to assess proangiogenic and antiangiogenic activities that encompass the key events in angiogenesis, i.e. matrix degradation, migration, proliferation and morphogenesis (Tab. 1.3.1).
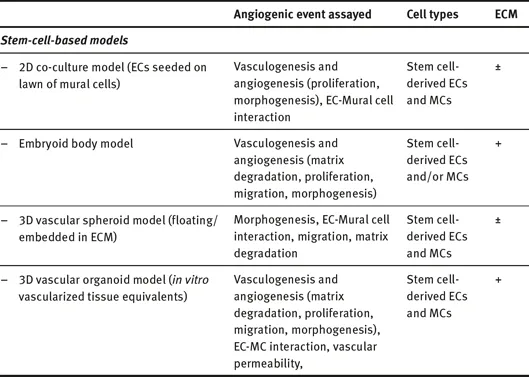
Tab. 1.3.1:In vitro assays to assess vasculogenesis and angiogenesis.
ECs = endothelial cells, ECM = extracellular matix, MCs = mural cells
1.3.1In vitro assays
1.3.1.1In vitro cell-based assays
Matrix degradation
Sprouting of new vessels requires degradation and proteolysis of basement membrane and connective tissue surrounding the parent blood vessel. Matrix degradation involves a family of proteases that includes matrix metalloproteinases (MMPs), other metalloproteinases, cathepsins, serine proteases and aminopeptidases. Activity of MMPs can be assessed using zymogen and matrix invasion assays. Zymogen assays are relatively inexpensive and provide the identities and relative levels of MMPs present in supernatants and lysates from endothelial cultures [16]. However, they do not allow differentiation of active and inactive MMPs or quantification of their relative expression levels.
Endothelial migration
Matrix degradation is followed by migration of ECs into the surrounding tissue e...