![]()
Chapter 1
Carbon Nanostructures: Covalent and Macromolecular Chemistry
Francesco Giacalone, Maa Ángeles Herranz, and Nazario Martín
1.1 Introduction
The aim of this introductory chapter is to bring to the attention of the readers the achievements made in the chemistry of carbon nanostructures and, mostly, in the chemistry of fullerenes, carbon nanotubes (CNTs), and the most recent graphenes. Since the discovery of fullerenes in 1985 and their further preparation in multigram amounts, the chemistry and reactivity of these molecular carbon allotropes have been well established. Actually, this chemical reactivity has been used as a benchmark for further studies carried out in the coming carbon nanotubes (single and multiple wall) and graphenes. Assuming that the fundamental chemistry of fullerenes is known and basically corresponds to that of typical electron-deficient alkenes, we have mainly focused on the chemistry of fullerene-containing polymers. In this regard, the combination of the unique fullerenes with the highly versatile polymer chemistry has afforded a new and interdisciplinary field in which the resulting architectures are able to exhibit unprecedented properties. The basic knowledge of this important topic of macromolecular chemistry of fullerenes nicely complements the following chapters devoted to their supramolecular chemistry.
The chemistry of CNTs, on the other hand, is considerable less developed than that of fullerenes, and most of their studied reactions are generally based on those previously studied on fullerenes. Therefore, despite the recent reviews and books published on CNTs, we feel that an introductory chapter describing the most significant solubilization/derivatization covalent and noncovalent methods should be helpful and welcome by the readers, and particularly to those nonexperts in the field. This same objective has been pursued for the most recent and planar graphenes. The available literature on the chemistry of these one-atom thickness carbon allotropes is considerably less developed. Therefore, some useful chemical procedures reported so far for the functionalization and solubilization of graphenes – thus allowing its manipulation and application for the construction of devices – have also been included at the end of the chapter.
1.2 Fullerene-Containing Polymers
Since the achievement of [60]fullerenes in ponderable amounts [1], its combination with macromolecular chemistry provided an opportunity to generate new fullerene-containing polymers, with potential for a broad scope of real applications since it merges C60 properties with the ease and versatile processability and handling of polymers. This approach prompted chemists to design and develop synthetic strategies aimed to obtain even more complex and fascinating novel fullerene-based architectures with unprecedented properties that have been recently reviewed 2. In fact, chemists are now able to tailor at will a polymeric backbone possessing C60 moieties in such way as to achieve peculiar properties of the final macromolecular material. In this way, block copolymer with well-defined donor–acceptor domains within the diffusion path of electron are created for solar devices [3], or water-soluble biocompatible and biodegradable polymers are designed in order to carry fullerene in circulation for photodynamic cancer therapy purposes 4. These recent achievements are only the tip of the iceberg of a growing field in which almost all the materials display outstanding properties such as optical limiting [5], or photoinduced electron transfer [6] just to name a few. In addition, polyfullerenes have been successfully employed as active materials not only in electroluminescent devices 7 but also in nonvolatile flash devices [8], and in membranes both for gas separation 9 and for proton exchange fuel cells [10].
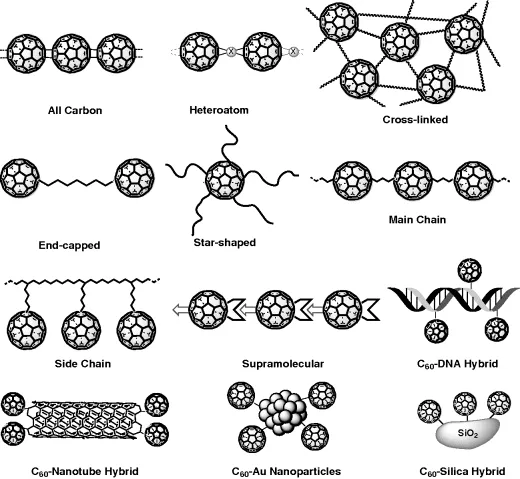
The many types of polymeric fullerene derivatives may be classified according to their structural features. As a criterion for the classification, polyfullerenes can be ordered as a function of their increasing chemical complexity and the difficulty to synthesize them, and other interesting classes of nondiscrete multifullerene-containing hybrid materials may also be included in this classification (Figure 1.1).
All-fullerene polymers are specifically those materials or structures that are constituted exclusively by fullerene units covalently linked to each other 10. These “intrinsic polymers” are prepared simply by exposing pure fullerene to visible light [12], high pressure [13], electron beam [14], and plasma irradiation 15 without control or care for the final structure. Recently, it has been shown that unbound and bound states of C60 molecules can be reversibly controlled at the single-molecule level in ultrathin films of all-C60 polymers using a tip of a scanning tunneling microscope at RT, thus allowing single-molecule-level topochemical digital data [16].
Heteroatom-containing polymers have metals or elements other than carbon inserted in between two C60 moieties. In 1994, Forró discovered that the fulleride phases AC60 (A: K, Rb, Rb or Cs), undergo [2 + 2] cycloadditions producing polyfullerenes with alkali metals in the crystal voids 17. For the organometallic polymers, the metal doping of C60 leads to the formation of the corresponding charge transfer polymer [18]. Several different metals have been employed in the polymerization with fullerenes, but among them palladium led to the most promising copolymers with outstanding properties [19]. In fact, the polymer (C60Pd3)n has been able to catalyze heterogeneous hydrogenation reactions of alkenes [20]. Very recently, 1D “zigzag” polymers have been achieved by mixing fullerene with the oxidizing superacid AsF5 [21]. As a result, a polyfullerenium salt in the solid state has been prepared, with C602+ units connected by an alternating sequence of four-membered carbon rings ([2 + 2] cycloaddition) and single C−C bonds stabilized by ASF6− anions in the lattice.
Cross-linked C60 polymers are synthesized from random and quick reactions that proceed in three dimensions with the help of the fullerene topology. Nevertheless, some extent of control of the addition reactions to the 30 fullerene double bonds is required in order to avoid a drastic intractability of the final products. This class of polymers can be prepared by four main pathways (Figure 1.2):
a. C60 or a C60 derivative and a monomer are mixed together and allowed to react randomly.
b. A multisubstituted C60 derivative is homopolymerized in three dimensions.
c. A preformed polymer properly functionalized at the end termini is allowed to react with C60 or a multisubstituted C60 derivative.
d. Polymers endowed with pending reacting moieties are allowed to react with C60.
Although cross-linked polymers are rather intractable materials, even in this family of polyfullerenes there exist some interesting and useful examples: random fullerene-containing polystyrenes (PS) 22 and poly(methylmethacrylate) [23] showed outstanding nonlinear optics. Nevertheless, cross-linked fullerene-containing polyurethanes displayed third-order NLO response with one–two orders of enhancement in comparison with other C60 derivatives 24. But the very “revenge” of this family against all the other processable C60 polymers stems from a novel approach described recently by Cheng and Hsu, which will create an organic photovoltaics revolution [25]. They generated in situ a robust cross-linked C60 pol...