![]()
1
From Jack Beans to Designer Genes
1.1 Introduction
Enzymes are giant macromolecules which catalyse biochemical reactions. They are remarkable in many ways. Their three-dimensional structures are highly complex, yet they are formed by spontaneous folding of a linear polypeptide chain. Their catalytic properties are far more impressive than synthetic catalysts which operate under more extreme conditions. Each enzyme catalyses a single chemical reaction on a particular chemical substrate with very high enantioselectivity and enantiospecificity at rates which approach “catalytic perfection”. Living cells are capable of carrying out a huge repertoire of enzyme-catalysed chemical reactions, some of which have little or no precedent in organic chemistry. In this book I shall seek to explain from the perspective of organic chemistry what enzymes are, how they work, and how they catalyse many of the major classes of enzymatic reactions.
1.2 The discovery of enzymes
Historically, biological catalysis has been used by mankind for thousands of years, ever since fermentation was discovered as a process for brewing and bread-making in ancient Egypt. It was not until the 19th century A.D. however that scientists addressed the question of whether the entity responsible for processes such as fermentation was a living species or a chemical substance. In 1897 Eduard Buchner published the observation that cell-free extracts of yeast containing no living cells were able to carry out the fermentation of sugar to alcohol and carbon dioxide. He proposed that a species called “zymase” found in yeast cells was responsible for fermentation. The biochemical pathway involved in fermentation was subsequently elucidated by Embden and Meyerhof – the first pathway to be discovered.
The exquisite selectivity of enzyme catalysis was recognised as early as 1894 by Emil Fischer, who demonstrated that the enzyme which hydrolyses sucrose, which he called “invertin”, acts only upon α-D-glucosides, whereas a second enzyme “emulsin” acts only upon β-D-glucosides. He deduced that these two enzymes must consist of “asymmetrically built molecules”, and that “the enzyme and glucoside must fit each other like a lock and key to be able to exert a chemical influence upon each other”. Fischer's “lock and key” hypothesis remained a powerful metaphor of enzyme action for many years. The crystallisation in 1926 of the enzyme urease (see Figure 1.1) from Jack beans by Sumner proved beyond doubt that biological catalysis was carried out by a chemical substance.
The recognition that biological catalysis is mediated by enzymes heralded the growth of biochemistry as a subject, and the elucidation of the metabolic pathways catalysed by enzymes. Each reaction taking place on a biochemical pathway is catalysed by a specific enzyme. Without enzyme catalysis the uncatalysed chemical process would be too slow to sustain life. Enzymes catalyse reactions involved in all facets of cellular life: metabolism (the production of cellular building blocks and energy from food sources); biosynthesis (how cells make new molecules); detoxification (the breakdown of toxic foreign chemicals); and information storage (the processing of deoxyribonucleic acids).
In any given cell there are present several thousand different enzymes, each catalysing its specific reaction. How does a cell know when it needs a particular enzyme? The production of enzymes, as we shall see in Chapter 2, is controlled by a cell's DNA, both in terms of the specific structure of a particular enzyme and the amount which is produced. Thus different cells in the same organism have the ability to produce different types of enzymes and to produce them in differing amounts according to the cell's requirements.
Since the majority of the biochemical reactions involved in cellular life are common to all organisms, a given enzyme will usually be found in many or all organisms, albeit in different forms and amounts. By looking closely at the structures of enzymes from different organisms which catalyse the same reaction, we can in many cases see similarities between them. These similarities are due to the evolution and differentiation of species by natural selection. So by examining closely the similarities and differences of an enzyme from different species we can trace the course of molecular evolution, as well as learning about the structure and function of the enzyme itself.
Recent developments in biochemistry, molecular biology and X-ray crystallography now allow a far more detailed insight into how enzymes work at a molecular level. We now have the ability to determine the amino acid sequence of enzymes with relative ease, whilst the technology for solving the three-dimensional structure of enzymes is developing apace. We also have the ability to analyse their three-dimensional structures using molecular modelling and then to change the enzyme structure rationally using site-directed mutagenesis. We are now starting to enter the realms of enzyme engineering, where by rational design we can modify the genes encoding specific enzymes, creating the “designer genes” of the title. These modified enzymes could in future perhaps be used to catalyse new types of chemical reactions, or via gene therapy to correct genetic defects in cellular enzymes which would otherwise lead to human diseases.
1.3 The discovery of coenzymes
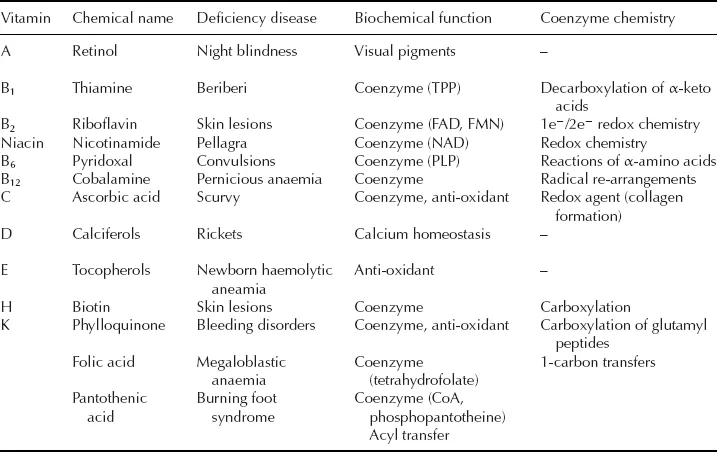
At the same time as the discovery of enzymes in the late 19th and early 20th centuries, a class of biologically important small molecules was being discovered which had remarkable properties to cure certain dietary disorders. These molecules were christened the vitamins, a corruption of the phrase “vital amines” used to describe their dietary importance (several of the first-discovered vitamins were amines, but this is not true of all the vitamins). The vitamins were later found to have very important cellular roles, shown in Table 1.1.
Table 1.1 The vitamins.
The first demonstration of the importance of vitamins in the human diet took place in 1753. A Scottish naval physician James Lind found that the disease scurvy, prevalent amongst mariners at that time, could be avoided by deliberately including green vegetables or citrus fruits in the sailors’ diets. This discovery was used by Captain James Cook to maintain the good health of his crew during his voyages of exploration in 1768–1776. The active ingredient was elucidated much later as vitamin C, ascorbic acid.
The first vitamin to be identified as a chemical substance was thiamine, lack of which causes the limb paralysis beriberi. This nutritional deficiency was first identified in the Japanese Navy in the late 19th century. The incidence of beriberi in sailors was connected with their diet of polished rice by Admiral Takaki, who eliminated the ailment in 1885 by improvement of the sailors’ diet. Subsequent investigations by Eijkman identified a substance present in rice husks able to cure beriberi. This vitamin was subsequently shown to be an essential “cofactor” in cellular decarboxylation reactions, as we shall see in Chapter 7.
Over a number of years the family of vitamins shown in Table 1.1 was identified and their chemical structures elucidated. Some like vitamin C have simple structures, whilst others like vitamin B12 have very complex structures. It has taken much longer to elucidate the molecular details of their biochemical mode of action. Many of the vitamins are in fact co-enzymes: small organic co-factors which are used by certain types of enzyme in order to carry out particular classes of reaction. Table 1.1 gives a list of the co-enzymes that we are going to encounter in this book.
1.4 The commercial importance of enzymes in biosynthesis and biotechnology
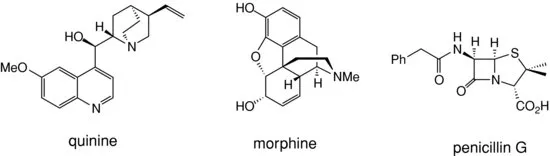
Many plants and micro-organisms contain natural products which possess potent biological activities. The isolation of these natural products has led to the discovery of many biologically active compounds such as quinine, morphine, and penicillin (see Figure 1.2) which have been fundamental to the development of modern medicine.
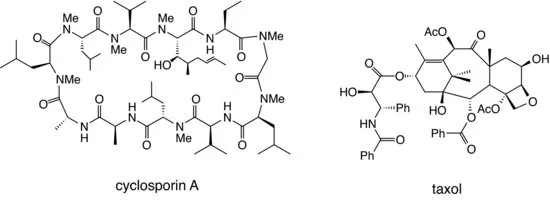
The process of natural product discovery continues today, with the recent identification of important compounds such as cyclosporin A, a potent immunosuppressant which has dramatically reduced the rejection rate in organ transplant operations; and taxol, an extremely potent anticancer drug isolated from yew bark (see Figure 1.3).
Many of these natural products are structurally so complex that it is not feasible to synthesise them in the laboratory at an affordable price. Nature, however, is able to bio-synthesise these molecules with apparent ease using enzyme-catalysed biosynthetic pathways. Hence there is considerable interest in elucidating the biosynthetic pathways for important natural products and using the enzymes to produce natural products in vitro. One example of this is the industrial production of semi-synthetic penicillins using a naturally occurring enzyme penicillin acylase (see Figure 1.4). Penicillin G which is obtained from growing Penicillium mould has certain clinical disadvantages; enzymatic deacylation and chemical re-acylation give a whole range of “semi-synthetic” penicillins which are clinically more useful.
The use of enzyme catalysis for commercial applications is an exciting area of the biotechnology...