
eBook - ePub
Surfactants in Cosmetics
Martin Rieger, Martin Rieger
This is a test
- 658 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Surfactants in Cosmetics
Martin Rieger, Martin Rieger
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
""Second Edition provides a thorough, up-to-date treatment of the fundamental behavior of surface active agents in solutions, their interaction with biological structures from proteins and membranes to the stratum corneum and epidermis, and their performance in formulations such as shampoos, dentifrice, aerosols, and skin cleansers.
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Surfactants in Cosmetics als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Surfactants in Cosmetics von Martin Rieger, Martin Rieger im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Scienze fisiche & Chimica. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
1
Surfactant Chemistry and Classification
MARTIN M. RIEGER Consultant, M & A Rieger Associates, Morris Plains, New Jersey
I. Introductory Comments
A. Definitions and structural requirements
B. Utility and selection of surfactants in cosmetics
C. Classification
D. Nomenclature
II. Group Description
A. Amphoterics
B. Anionics
C. Cationics
D. Nonionics
References
1 Introductory Comments
A Definitions and Structural Requirements
The term surfactant is shorthand for the more cumbersome “surface active agent.” Surfactants as a group have the ability to modify the interface between various phases. Their effects on the interface are the result of their ability to orient themselves in accordance with the polarities of the two opposing phases. Thus the polar (hydrophilic) part of the surfactant molecule can be expected to be oriented toward the more polar (hydrophilic) phase at a given interfacial contact site. Similarly, the nonpolar (lipophilic) portion of the surfactant molecule should contact the nonpolar (lipophilic) phase. Each surfactant molecule has a tendency to reach across (bridge) the two phases, and such substances have, therefore, also been called amphiphilic.
One of the prerequisites for an amphiphilic molecule is possession of at least one polar and at least one essentially nonpolar portion. The orientation of a 1,2-dodecanediol molecule at a mineral-oil/water interface is readily predictable from the preceding discussion, but the positioning of 1,12-dodecanediol at a similar interface is not as obvious; it would be expected to be different and more complex than that of the 1,2-isomer. Despite their chemical similarity, the surfactant activities of these two compounds can be expected to be different. It is apparent from this that a surfactant’s behavior or utility, e.g., as an emulsion stabilizer, is unrelated to its empirical formula. Instead, a surfactant’s spatial configuration, i.e., the molecule’s structure, plays a critical role in determining its application in cosmetics.
B Utility and Selection of Surfactants in Cosmetics
Those who require and use surfactants tend to define surfactants on the basis of performance. Regardless of diverse theoretical considerations, practicing cosmetic formulators have developed a usage classification that they find practical in their day-to-day activities. As a rule, a surfactant is soluble in at least one of the contacting phases and is used to perform one or more of the following tasks:
Clean (Detergency),
Wet,
Emulsify,
Solubilize,
Disperse, or Foam.
Surfactants are useful for creating a wide variety of dispersed systems, such as suspensions and emulsions. They cleanse and solubilize and are required not only during manufacture but are also essential for maintaining an acceptable level of physical stability of thermo-dynamically unstable systems, such as emulsions. Few modem cosmetic products exist that do not depend on one or more surfactants to create and maintain their desired characteristics.
It is the practitioner’s responsibility to select one or more surfactants that can perform the task at hand. As a result of prior experience, formulators usually can identify those surfactant structures that can be expected to be most useful for achieving the desired goal.
The cosmetic formulator’s choice of surfactants is more limited than that of the industrial chemist. Some of the criteria influencing selection are briefly noted below:
Safety—Adverse reactions to any surfactant used in a finished cosmetic must be minimized.
Odor and Color—Odoriferous or deeply colored surfactants can affect the esthetics of a finished product and should be avoided.
Purity—Impurities present in some surfactants may make the surfactant unacceptable for cosmetic use.
Despite these and other limitations and the obvious requirement of cost, the cosmetic chemist must make a selection from about 2000 different commercially available surfactants.
The selection for the specific formulation task requires insight into the general chemical characteristics of surfactants (this chapter) and an understanding of the physichochemical behavior of these amphiphiles (Chapter 2).
C Classification
Classification or categorization of the thousands of different surfactants on the basis of generally recognized principles is clearly desirable. Thus it would appear practical to base such a scheme on the surfactant’s functionality. Creating groupings based on such functional groups could in all likelihood be made without regard to commonly accepted chemical or physical characteristics. A typical functional scheme was developed in the CTFA (Cosmetic Ingredient Handbook) [1] by creating six functional categories for surfactants:
Surfactants, Cleansing Agents
Surfactants, Emulsifying Agents
Surfactants, Foam Boosters
Surfactants, Hydrotropes
Surfactants, Solubilizing Agents
Surfactants, Suspending Agents
An entirely different means for classification might be based on the nature of the hydrophobic portions of surfactants. Such a classification would create groups based on the presence of hydrophobes derived from paraffinic, olefinic, aromatic, cycloaliphatic, or heterocyclic hydrophobes. This type of classification could be of particular interest to specialists who may wish to compare substances on the basis of physiological effects related to the origin of the lipophilic constituents.
The most useful and widely accepted classification is based on the nature of the hydrophilic segment of the surfactant molecules. This classification system has universal acceptance and has been found to be practical throughout the surfactant industry. This approach creates four large groups of chemicals: amphoterics, anionics, cationics, and nonionics. This system categorizes surfactants on the basis of their ionic or nonionic character, does not consider differences in the hydrophobic (nonpolar) segment, and ignores functionality.
It is common practice to depict surfactant molecules as ball and stick figures:
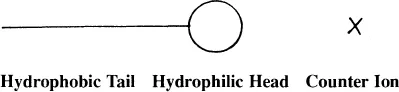
In this cartoon, the hydrophobe is represented by a stick; the ball represents the hydrophilic grouping, which may carry a positive and/or a negative charge or no charge; X represents the counter ion required for electro-neutrality of the molecule.
D Nomenclature
The nomenclature of surfactants can become very complex and confusing. For the purpose of labeling of cosmetics in accordance with U.S. regulation, the Cosmetics, Toiletry and Fragrance Association has created names for cosmetic ingredients. It is likely that these names will soon be accepted in many other countries in the hope that a worldwide agreement on this INCI* nomenclature can be reached between governmental regulatory agencies and the trade associations concerned with cosmetics.
Rules for creating these names are included in the International Cosmetic Ingredient Dictionary [2]. The names are intended to be descriptive for laypersons as well as the more technically oriented. The assigned names are not as precise as the names assigned by Chemical Abstracts and eliminate the need for using proprietary trade names. The INCI names are used in this chapter wherever possible.
Some abbreviations used in the text are identified below:

II Group Description
A Amphoterics
Surfactants are classified as amphoteric if—and only if—the charge(s) on the hydrophilic head change as a function of pH. Such surfactants must carry a positive charge at low pH and a negative charge at high pH and may form internally neutralized ionic species (zwitterions) at an intermediate pH. These features of amphoterics are illustrated below with the behavior of lauramino-propionic acid at various pH levels:
[R—NH2—CH2—CH2—COOH]+ X-
Low pH: The surfactant molecule is a cation.
[R—NH2—CH2—CH2—COO]+-
Intermediate pH: The surfactant molecule is a zwitterion.
[R—NH—CH2—CH2—COO]- C+
High pH: The surfactant molecule is an anion.
In this example, R represents the lauryl alkyl group, while X- and C+ are the required counter ions. The behavior of this substance must be compared with that of lauryl betaine:
[R—N (CH3 )2—CH2—COOH]+ X-
Low pH: The surfactant molecule is a cation.
[R—N (CH3 )2—CH2—COO]+-
Intermediate pH: The surfactant molecule may be a zwitterion.
[R—N(CH3)2—CH2—COO]+−
High pH: The surfactant molecule is a cation and an anion.
Lauryl betaine contains a quaternary nitrogen atom regardless of pH. The ionization of the carboxylic acid group is, however, pH dependent, and internal compensation is possible. Lauryl betaine is properly cla...
Inhaltsverzeichnis
- Cover Page
- Title Page
- Copyright Page
- Contents of the Second Edition
- Preface to the Second Edition
- Preface to the First Edition
- Contents of the First Edition
- Contributors
- 1. Surfactant Chemistry and Classification
- 2. Physical Properties of Surfactants Used in Cosmetics
- 3. The Analysis of Surfactants in Cosmetics
- 4. Principles of Emulsion Formation
- 5. Emulsifier Selection/HLB
- 6. Multiple Emulsions in Cosmetics
- 7. Multiphase Emulsions
- 8. Stability of Emulsions
- 9. Phase Inversion in Emulsions: CAPICO—Concept and Application
- 10. Solubilization in Cosmetic Systems
- 11. Selection of Solubilizers
- 12. Liposomes and Niosomes
- 13. Surfactants for Skin Cleansers
- 14. Cleansing Bars for Face and Body: In Search of Mildness
- 15. Topical Antibacterial Wash Products
- 16. Hair Cleansers
- 17. Surfactants in Dental Products
- 18. In Vitro Interactions: Biochemical and Biophysical Effects of Surfactants on Skin
- 19. Surfactant Mildness
- 20. Surfactant Effects on Skin Barrier
- 21. Bioengineering Techniques for Investigating the Effects of Surfactants on Skin
- 22. Skin Penetration Enhancement by Surfactants
- 23. Human In Vivo Methods for Assessing the Irritation Potential of Cleansing Systems
- 24. The Challenge of Using the "Inarticulate" Consumer as an R & D Partner in Cosmetic Product Development
- 25. Toxicology of Surfactants Used in Cosmetics
- 26. Chemical Instability of Surfactants
- 27. Inactivation of Preservatives by Surfactants
- 28. Solubilization of Fragrances by Surfactants
- Index
Zitierstile für Surfactants in Cosmetics
APA 6 Citation
[author missing]. (2017). Surfactants in Cosmetics (2nd ed.). CRC Press. Retrieved from https://www.perlego.com/book/1496903/surfactants-in-cosmetics-pdf (Original work published 2017)
Chicago Citation
[author missing]. (2017) 2017. Surfactants in Cosmetics. 2nd ed. CRC Press. https://www.perlego.com/book/1496903/surfactants-in-cosmetics-pdf.
Harvard Citation
[author missing] (2017) Surfactants in Cosmetics. 2nd edn. CRC Press. Available at: https://www.perlego.com/book/1496903/surfactants-in-cosmetics-pdf (Accessed: 14 October 2022).
MLA 7 Citation
[author missing]. Surfactants in Cosmetics. 2nd ed. CRC Press, 2017. Web. 14 Oct. 2022.