
eBook - ePub
Carbon Nanotubes
Science and Applications
M. Meyyappan, M. Meyyappan
This is a test
Buch teilen
- 310 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Carbon Nanotubes
Science and Applications
M. Meyyappan, M. Meyyappan
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
Carbon nanotubes, with their extraordinary mechanical and unique electronic properties, have garnered much attention in the past five years. With a broad range of potential applications including nanoelectronics, composites, chemical sensors, biosensors, microscopy, nanoelectromechanical systems, and many more, the scientific community is more moti
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Carbon Nanotubes als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Carbon Nanotubes von M. Meyyappan, M. Meyyappan im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Naturwissenschaften & Industrielle & technische Chemie. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
1: Structures and Properties of Carbon Nanotubes
Jie Han
NASA Ames Research Center
0–8493–2111–5/05/$0.00+$1.50
© 2005 by CRC Press LLC
Since the discovery of carbon nanotubes (CNTs) by Iijima in 1991 [1], great progress has been made toward many applications, including, for example:
- Materials
- Chemical and biological separation, purification, and catalysis
- Energy storage such as hydrogen storage, fuel cells, and the lithium battery
- Composites for coating, filling, and structural materials
- Devices
- Probes, sensors, and actuators for molecular imaging, sensing, and manipulation
- Transistors, memories, logic devices, and other nanoelectronic devices
- Field emission devices for x-ray instruments, flat panel display, and other vacuum nanoelectronic applications
The advantages of these applications have been demonstrated, including their small size, low power, low weight, and high performance, and will be discussed in the following chapters. These applications and advantages can be understood by the unique structure and properties of nanotubes, as outlined below:
- Structures (Sections 1.1–1.3)
- Bonding: sp2 hybrid orbital allows carbon atoms to form hexagons and occasionally pentagons and pentagon units by in-plane σ bonding and out-of-plane π bonding.
- Defect-free nanotubes: these are tubular structures of hexagonal network with a diameter assmall as 0.4 nm. Tube curvature results in σ-π rehybridization or mixing.
- Defective nanotubes: occasionally pentagons and heptagons are incorporated into a hexagonal network to form bent, branched, coroidal, helical, or capped nanotubes.
- Properties (Sections 1.4–1.9)
- Electrical: electron confinement along the tube circumference makes a defect-free nanotube either semiconducting or metallic with quantized conductance whereas pentagons and heptagons will generate localized states.
- Optical and optoelectronic: direct band gap and one-dimensional band structure make nano-tubes ideal for optical applications with wavelength ranging possibly from 300 to 3000 nm.
- Mechanical and electromechanical: σ-π rehybridization gives nanotubes the highest Young’s modulus of over 1 TPa and tensile strength of over 100 GPa and remarkable electronic response to strain and metal-insulator transition.
- Magnetic and electromagnetic: electron orbits circulating around a nanotube give rise to many interesting phenomena such as quantum oscillation and metalinsulator transition.
- Chemical and electrochemical: high specific surface and σ-π rehybridization facilitate molecular adsorption, doping, and charge transfer on nanotubes, which, in turn, modulates electronic properties.
- Thermal and thermoelectric: inherited from graphite, nanotubes display the highest thermal conductivity while the quantum effect shows up at low temperature.
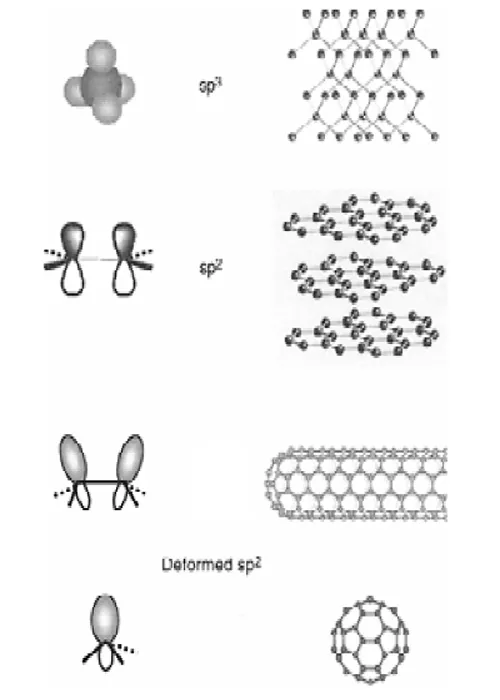
FIGURE 1.1 Bonding structures of diamond, graphite, nanotubes, and fullerenes: when a graphite sheet is rolled over to form a nanotube, the sp2 hybrid orbital is deformed for rehybridization of sp2 toward sp3 orbital or σ-π bond mixing. This rehybridization structural feature, together with π electron confinement, gives nanotubes unique, extraordinary electronic, mechanical, chemical, thermal, magnetic, and optical properties.
1.1 Bonding of Carbon Atoms
To understand the structure and properties of nanotubes, the bonding structure and properties of carbon atoms are discussed first. A carbon atom has six electrons with two of them filling the 1s orbital. The remaining four electrons fill the sp3 or sp2 as well as the sp hybrid orbital, responsible for bonding structures of diamond, graphite, nanotubes, or fullerenes, as shown in Figure 1.1.
In diamond [2], the four valence electrons of each carbon occupy the sp3 hybrid orbital and create four equivalent s covalent bonds to connect four other carbons in the four tetrahedral directions. This three-dimensional interlocking structure makes diamond the hardest known material. Because the electrons in diamond form covalent s bonds and no delocalized p bonds, diamond is electrically insulating. The electrons within diamond are tightly held within the bonds among the carbon atoms. These electrons absorb light in the ultraviolet region but not in the visible or infrared region, so pure diamond appears clear to human eyes. Diamond also has a high index of refraction, which makes large diamond single crystals gems. Diamond has unusually high thermal conductivity.
In graphite [3], three outer-shell electrons of each carbon atom occupy the planar sp2 hybrid orbital to form three in-plane s bonds with an out-of-plane p orbital (bond). This makes a planar hexagonal network. van der Waals force holds sheets of hexagonal networks parallel with each other with a spacing of 0.34 nm. The s bond is 0.14 nm long and 420 kcal/mol strong in sp2orbital and is 0.15 nm and 360 kcal/mol in sp3 configuration. Therefore, graphite is stronger in-plane than diamond. In addition, an outof- plane p orbital or electron is distributed over a graphite plane and makes it more thermally and electrically conductive. The interaction of the loose p electron with light causes graphite to appear black. The weak van der Waals interaction among graphite sheets makes graphite soft and hence ideal as a lubricant because the sheets are easy to glide relative to each other.
A CNT can be viewed as a hollow cylinder formed by rolling graphite sheets. Bonding in nanotubes is essentially sp2. However, the circular curvature will cause quantum confinement and s-p rehybridization in which three s bonds are slightly out of plane; for compensation, the p orbital is more delocalized outside the tube. This makes nanotubes mechanically stronger, electrically and thermally more conductive, and chemically and biologically more active than graphite. In addition, they allow topological defects such as pentagons and heptagons to be incorporated into the hexagonal network to form capped,bent, toroidal, and helical nanotubes whereas electrons will be localized in pentagons and heptagons because of redistribution of p electrons. For convention, we call a nanotube defect free if it is of only hexagonal network and defective if it also contains topological defects such as pentagon and heptagon or other chemical and structural defects.
Fullerenes (C60) are made of 20 hexagons and 12 pentagons [4]. The bonding is also sp2, although once again mixed with sp3 character because of high curvature. The special bonded structures in fullerene molecules have provided several surprises such as metalinsulator transition, unusual magnetic correlations, very rich electronic and optical band structures and properties, chemical functionalizations, and molecular packing. Because of these properties, fullerenes have been widely exploited for electronic, magnetic, optical, chemical, biological, and medical applications.
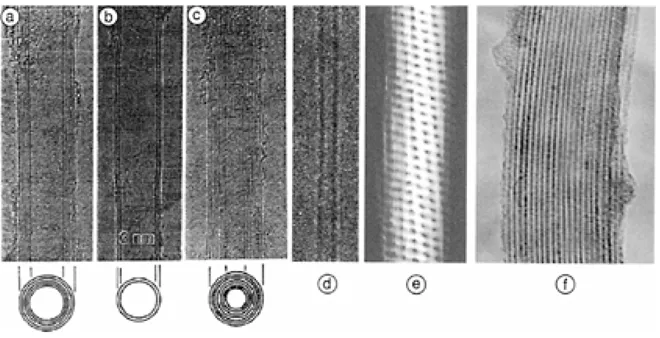
FIGURE 1.2 Homogeneous nanotubes of hexagonal network: TEM images (a),(b),and (c) for three multiwalled nanotubes (MWNTs) first discovered by Iijima in 1991 [1]; TEM image (d) for a single-wall nanotube (SWNT) first discovered by Iijima et al. in 1993 [5, 6], an atomic resolution STM image (e) for a SWNT; and a TEM image (f) for a SWNT rope first reported in 1996 by Thess et al. [7]. (Figures 1.2a and 1.2b are from Iijima, S., Nature, 354.56, 1991; Figure 1.2d is from Iijima, and Ichihashi, Nature, 363, 603, 1991; Figure 1.2f is from Thess et al, Science, 273, 483, 1996.)
1.2 Defect-Free Nanotube
There has been a tremendous amount of work studying defect-free nanotubes, including single or multiwalled nanotubes (SWNTs or MWNTs). A SWNT is a hollow cylinder of a graphite sheet whereas a MWNT is a group of coaxial SWNTs. SWNT was discovered in 1993 [5, 6], 2 years after the discovery of MWNT [1]. They are often seen as straight or elastic bending structures individually or in ropes [7] by transmission electron microscopy (TEM), scanning electron microscopy (SEM), atomic force microscopy (AFM), and scanning tunneling microscopy (STM). In addition, electron diffraction (EDR), x-ray diffraction (XRD), Raman, and other optical spectroscopy can be also used to study structural features of nanotubes. These characterization techniques will be discussed in detail in Chapter 5. Figure 1.2 shows an STM image with atomic resolution of a single SWNT from which one can see the hexagonal structural feature and TEM images of a SWNT rope and a few MWNTs.
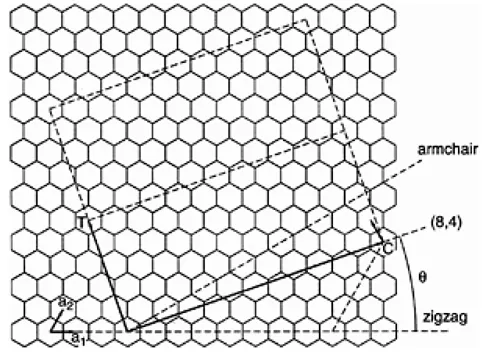
FIGURE 1.3 A nanotube (n,m) is formed by rolling a graphite sheet along the chiral vector C=na1+ma2 on the graphite where a1 and a2 are graphite lattice vector. The nanotube can also be characterized by the diameter |C| and the chiral angle θ is with respect to the zigzag axis, θ=0°. The diagram is constructed for a(8,4) nanotube.
A SWNT can be visualized as a hollow cylinder, formed by rolling over a graphite sheet. It can be uniquely characterized by a vector C in terms of a set of two integers (n,m) corresponding to graphite vectors a1 and a2 (F...