![]()
Chapter 1
Fundamental Concepts of Development of Genetically Engineered Plants
At all stages of social development, ensuring the availability of food has been a prerequisite condition for survival of mankind, so the most advanced and efficient ways have been used to achieve this goal. Plant varieties and animal breeds used in agriculture have been produced through a centuries-old selection process targeted to enhance crop yield and animal productivity, adaptation to environment, increase of nutritional value, improvement of flavor, appearance, etc. By accumulation of knowledge, the researchers more and more efficiently manipulated the genome of the selected species, trying to obtain the desired traits in the shortest period of time. However, the capabilities of the methods were limited because the boundaries of selection were limited to the genome of a single species in each case. Acquisition of novel traits became possible only after overcoming interspecies barriers through the use of genetic engineering. In essence, genetic engineering uses traditional selection intended to improve the genotype of economically valuable crops, but it employs far more precise methods that significantly shorten the time needed to generate plants with desired traits [1,14,22,24,45].
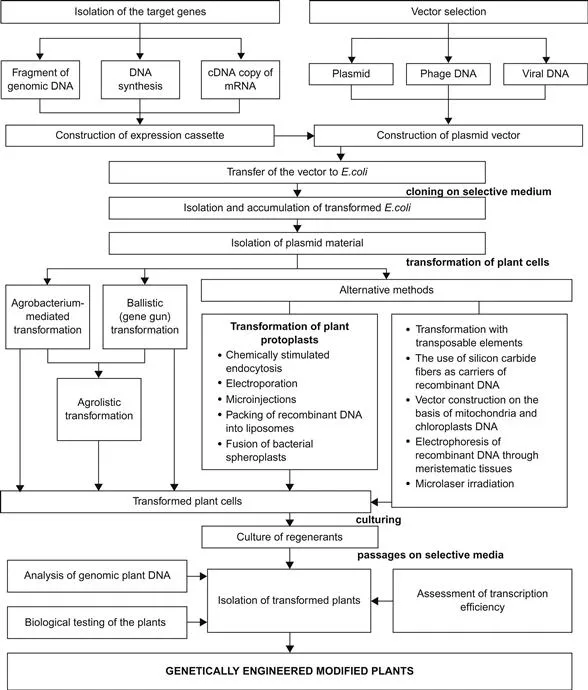
At present, genetic engineering makes it possible to transfer the genes from one organism to another. Specifically, the methods of genetic engineering include synthesis of the genes, isolation of individual genes or hereditary structures from the cells, followed by rearrangement, copying, and multiplication of the isolated or synthesized genes or genetic structures, and integration of various genomes within a cell [1–3,24]. The process of generating genetically modified (GM) plants consists of several stages. The basic stages of this technique are: isolation of the target genes, insertion of the genes into a transfer vector, transformation of the plant cells, confirmation of transformation by molecular characterization of the inserted cassette, demonstrating the function of the target gene, and finally regeneration of the whole plant from the transformed cells (Figure 1.1) [5,7,61].
Figure 1.1 Engineering of transgenic plants.
A genetic vector is a DNA molecule used in genetic engineering to transfer the genes from the donor to the recipient organism. A vector can be composed of a small extrachromosomal element (plasmid, phage, or viral DNA). As a rule, plasmid vectors are used to transform the plants. The most widely used are combinations, where the role of intermediate host is given to a strain of E. coli and its plasmid, while Agrobacterium tumefaciens and its plasmid are the final hosts [39,50,53].
At the first stage of recombinant DNA production, insertions suitable for connection with the vector molecule are prepared. There are three ways to obtain such insertions:
■ from genomic DNA fragmented with the use of restriction endonucleases or physical methods (such as ultrasound sonication);
■ by synthesis of DNA fragments obtained by chemical or enzymatic methods or combination of thereof;
■ from DNA segments (complementary DNA, cDNA) obtained through the use of enzymatic copying of RNA matrix in vitro[26,29,32].
In most cases, the target gene is modified, despite the universal character of the genetic code, because the codons encoding the same amino acids in prokaryotes and eukaryotes do differ significantly. Modification is necessary to exclude the gene from the sequences that potentially could be the sites of polyadenylation (premature termination of transcription) or could destabilize mRNA. In addition, the structural region of prokaryotic genes can incorporate undesirable signal sequences recognizable by splicing or degradation enzymes at mRNA level. The presence of such hidden signals results in a dramatic decrease of gene expression in the plant, so they are usually eliminated by the targeted substitution of the nucleotides [4,9,11]. There is a number of standard techniques to perform directed substitutions in the coding sequences based on the polymerase chain reaction (PCR) method. Replacement of codons does not affect the primary protein structure, although expression of a gene can be amplified up to 300-fold [42].
To express the target gene properly in plant cells, it should be placed under control of the corresponding regulatory elements that efficiently ensure transcription: promoters, transcription initiation sites, and terminators. Among the eukaryotic organisms, these elements are extremely conservative and universal. As a rule, plant cells correctly express foreign genes originated not only from the plants of different species, but also the genes of mammals, yeast, and other eukaryotes. Constitutive promoters are employed to obtain the product of the corresponding gene in significant amounts during the entire life of the plant. Examples of such promoters are the cauliflower mosaic virus 35 S promoter, the Agrobacterium nopaline synthase (nos1) promoter for dicotyledonous plants, the promoters of the maize alcohol dehydrogenase gene (adh) and the rice actin1 gene (act) for monocotyledonous plants. Among the presently isolated promoters, the cauliflower mosaic virus 35 S promoter has proven to be the most effective, so it is widely used for expression of target genes [11,31]. In addition to constitutive promoters, there are specific promoters active in particular tissues, cells, or at certain stages of plant ontogenesis. An example is the potato patatin gene promoter, which works in tubers only [44]. Inducible promoters that are activated by factors such as temperature, illumination, and chemical agents are being studied. Inducible promoters are rather attractive both for fundamental and applied research—specifically, in biotechnology where they can induce gene expression within a given period of time [33,40,55,61].
Therefore, the insert or expression cassette of the plasmid vector is a group of functionally related DNA fragments composed of a highly active promoter, immediately followed by the target gene and transcription terminator.
After the plasmids with inserts are obtained, it is possible to construct the plasmid vectors by digestion of the plasmid with the corresponding restriction enzymes followed by ligation with the insert. The conditions of vector ligation depend on the character of terminal parts of the vector and the insert, which can be either sticky (complementary single-strand DNA fragments at the opposite ends of the double-strand molecule) or blunt (the ends of the double-strand DNA molecule terminated with a bound pair of the complementary bases). The sticky ends of the vector and insert are connected by DNA-ligase under conditions promoting the development of hydrogen bonds between the complementary nucleotide ends. To fuse the blunt end, DNA-ligase and the fragments should be available in enhanced concentrations, because affinity of this enzyme to the blunt end is low. In addition to ligation of vector with insert, any of these fragments can bind its counterpart, producing vector-vector or insert-insert complexes and thereby decreasing the output of recombinant molecules [15].
The resulting plasmid vector is transferred into the host cell for cloning and amplification. The basic tool of molecular cloning is the two-component system of the compatible combination of vector and host, because efficient replication is possible only under optimal conditions for the plasmid vector, which employs not only the metabolites, enzymes, and other proteins of the host cell, but also its protein synthesis apparatus [24,53,63].
Penetration of isolated DNA molecules into the live cells of E. coli (transfection) proceeds most efficiently when permeability of the membrane increases—for example, due to its local breakage. The breaks in the cell membrane can be produced by exposure of the cells to certain chemicals or by the direct effect of electric current (electroporation). Thereafter the transfer of plasmid DNA is performed during few minutes (transformation efficiency determined by the number of transformed cells per 1 µg introduced DNA is 107–108 or 106–109, correspondingly) [3].
A prerequisite condition of successful cloning is the possibility to separate all the transfected E. coli cells. Usually the plasmid vectors incorporate marker genes introducing to the host cells a phenotype which can be easily used in the selection process because it indicates the presence of this vector. For example, cells sensitive to a certain antibiotic or toxin can be used in combinations with the vectors, which incorporate the genes conferring resistance to these agents. By culturing the microorganisms under conditions revealing the dependence of these microorganisms on the vector genes, it becomes possible to identify, select, and multiply the cell harboring the desired genetic material [64].
Production of recombinant DNA in amounts necessary to modify the plant genome is the final prerequisite prior to the key stage of development of a GM plant. There is a number of methods to transform the plant genome, such as Agrobacterium-mediated transformation, ballistic (gene gun) transfer, injection of genes into plant protoplasts, and some alternative approaches (Figure 1.1) [3,7,58,61]. Efficient and reliable transfer of vector DNA into the plant protoplasts can be performed by electroporation, microinjection, DNA packing in liposomes, fusion of bacterial spheroplasts, and chemically induced endocytosis [7]. Protoplasts (plant cells deprived of cell wall after exposure to cellulases) are the most suitable recipients of recombinant DNA, because they are bacteria-like systems where each cell assumes a competent state and possesses pronounced proliferative ability. In comparison with multicellular transformations, the use of protoplasts significantly decreases losses during selection of modified cells [58]. The alternative ways of genetic transformation are mostly experimental. One of them is the design of vectors based on plant cell organelles; another produces the genetic transformation with the use of transposed elements. In addition, there are the techniques to modify the plant genome that use silicon fibers 0.6 µm in diameter and 10–80 µm in length as carriers of the recombinant DNA and transport it across the meristematic tissues by electrophoresis, or to alter it by microlaser irradiation making microscopic pores in cell walls and membranes as a preliminary step to incubation of the perforated cells in solutions with vector DNA [7,58,61].
Ideally, the transformation system should be simple, inexpensive, and efficient. However, despite a rather wide selection of methodological approaches, no method meets all of these requirements. Presently, the large-scale production of GMOs is based mostly on the agrobacterial and ballistic technique used to modify the plant genome [20,38,49].
Agrobacterium (A. tumefaciens and A. rhizogenes) belong to the Rhyzobium genus of bacteria with the characteristic ability to induce tumors (crown-gall disease) in many dicotyledonous plants. A fragment of agrobacterial tumor-inducing (Ti)-plasmid, called transfer DNA (T-DNA), is transferred into the genome of plant cells. The delivery of T-DNA is a unique natural process of genetic information exchange betw...