
eBook - ePub
Bioactive Food as Dietary Interventions for the Aging Population
Bioactive Foods in Chronic Disease States
Ronald Ross Watson,Victor R Preedy
This is a test
- 514 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Bioactive Food as Dietary Interventions for the Aging Population
Bioactive Foods in Chronic Disease States
Ronald Ross Watson,Victor R Preedy
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
Bioactive Food as Dietary Interventions for the Aging Population presents scientific evidence of the impact bioactive foods can have in the prevention and mediation of age related diseases. Written by experts from around the world, this volume provides important information that will not only assist in treatment therapies, but inspire research and new work related to this area.
- Focuses on the role of bioactive foods in addressing chronic conditions associated with aging and senescence
- Important information for developing research on this rapidly growing population representing an increasingly significant financial burden
- Documents foods that can affect metabolic syndrome and ways the associated information could be used to understand other diseases, which share common etiological pathways
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Bioactive Food as Dietary Interventions for the Aging Population als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Bioactive Food as Dietary Interventions for the Aging Population von Ronald Ross Watson,Victor R Preedy im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Technology & Engineering & Food Science. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
Thema
Food ScienceChapter 1
Antioxidant Supplementation in Health Promotion and Modulation of Aging
An Overview
1. Oxygen and Oxidative Stress
Antioxidants have become a necessity as a consequence of adaptation to life under aerobic conditions. Oxygen (or dioxygen, triplet O2) is strictly necessary for our energetic metabolism and evolution has found a way to increase its concentration in aqueous environments, and to transport it into our internal fluids by means of hemoglobin and other heme-containing proteins. Oxygen is needed for its oxidizing property, that is, oxidizing food (carbohydrates, lipids, and some amino acids) and using the released electrons to reduce NAD+ and oxidized flavins to NADH, FMNH2, and FADH2. These, in turn, are used in the mitochondria to produce adenosine triphosphate (ATP), again exploiting the oxidizing ability of O2 (that will be converted to H2O) as the driving force for the overall reaction. Incidentally, the oxidizing activity of oxygen (or its derivatives) sometimes goes out of control and results in so-called oxidative stress, which can lead to biological damage if not balanced by antioxidant defenses. Indeed, oxidative stress can be defined as the imbalance between generation of oxidating oxygen derivatives and antioxidant defenses, while the oxidative stress status (OSS) is a measure of such an imbalance (Halliwell and Gutteridge, 1999).
Reduction of oxygen to water using NADH in the inner membrane of the mitochondria is a spontaneous yet highly controlled process, occurring through a cascade of redox reactions called the electron transport chain, as exemplified in Figure 1.1. Such a sophisticated molecular machine is, however, not perfect and can leak electrons throughout the chain. Particularly, complexes I and III have been identified as the weak rings of the chain, due to the involvement of the intermediate semiquinone radical CoQH•, which can react with molecular oxygen to form superoxide radical anion (O2•¯), one of the most abundant reactive oxygen species (ROS) in biological systems (Finkel and Holbrook, 2000). Approximately 10–15% of the total oxygen intake is consumed in uncatalyzed chemical oxidation or by a variety of oxygenases and oxidases and not used for energetic metabolisms (in the mitochondria). This is a very relevant source of ROS, particularly the P450 superfamily of monooxygenase.
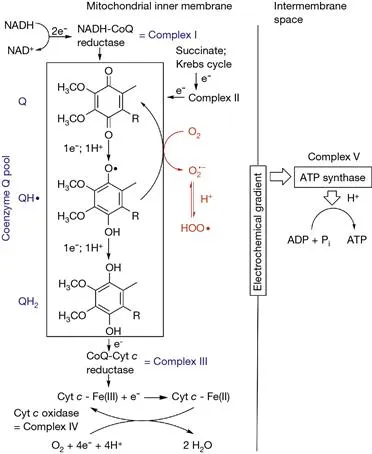
Figure 1.1 The mitochondrial electron-transport chain starts with the transfer of electrons from NADH to coenzyme Q (ubiquinone, CoQ) to form the reduced hydroquinone (CoQH2): although the overall reaction involves the transfer of two electrons, the intermediate one-electron reduced form, ubisemiquinone radical (CoQH•), is also present. Reduction of CoQ by NADH is controlled by NADH:coenzyme Q reductase called complex I. CoQ also accepts electrons from reduced flavoproteins generated by the Krebs cycle (complex II, including succinate dehydrogenase) and oxidation of fatty acids. CoQH2 in turn passes the electrons to coenzyme Q:cytochrome c reductase or complex III. Cytochromes are heme-proteins, and the electrons are used, one at a time, to reduce FeIII to FeII: Cyt-Fe3+ + e− → Cyt-Fe2+. The reaction then goes backward (to regenerate Cyt-Fe3+) in the next step, when the electron is passed over to the multienzyme cytochrome c oxidase (complex IV) that uses e electrons to reduce one molecule of O2 to two molecules of H2O. The movement of electrons through the cascade also induces a migration of H+ into the intermembrane space, and the energy associated to this electrochemical gradient is stored by ATP synthase (complex V) into ATP molecules. During the chain, electrons are transferred from CoQH• to O2 to form superoxide radicals.
Cytochrome P450 enzymes are involved in the oxidation of several compounds, including xenobiotics, and their expression is induced by the xenobiotics themselves, such as ethanol. Similar to cytochrome c, their operation, represented in Figure 1.2, is not error-proof and can lead to the formation of superoxide and hydrogen peroxide (H2O2). Hydrogen peroxide and superoxide radicals are also formed by several cytosolic oxidases, whose primary task appears to be indeed the formation of such species, which serve as both chemotactic factors and chemical messengers in a multitude of redox-sensitive regulatory processes within the cell.
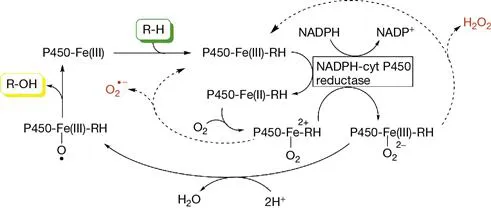
Figure 1.2 Hydroxylation of an organic substrate RH by P450 oxygenases. ROS production occurs as a side event.
As part of the inflammatory process, organic peroxides (ROOR) and hydroperoxides (ROOH) are formed in the arachidonic acid cascade. A very relevant source of oxidative stress comes from Fenton-type chemistry (Eq. 1.1), which occurs spontaneously to hydrogen peroxide and organic hydroperoxides in the presence of transition metal ions such as Fe2+ and Cu+ in solution, and leads to the formation of hydroxyl (HO•) and alkoxyl (RO•) radicals. Ionizing radiations or photochemical reactions in skin exposed to sunlight can be an additional source of reactive species.
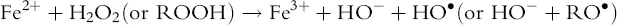
1.1 ROS, Reactive Nitrogen Species (RNS), and Free Radicals Involved in Oxidative Stress
Radicals have an unpaired electron in their outer (valence) electronic shell, which normally makes them highly unstable and reactive. They might be free radicals, that is, neutral, without a counterion, or radical ions (anion or cation). They may or may not be oxidizing species; this depends on their redox potential, on the reactivity of other molecules in the surroundings, and on the environment. ROS comprise both radical and molecular oxygen metabolites involved in oxidative damage to biomolecules, particularly superoxide radical (O2•−/HOO•), peroxyl radicals (ROO•), hydroxyl radicals (HO•), alkoxyl radicals (RO•), hydrogen peroxide (H2O2), alkyl hydroperoxides (ROOH), organic peroxides (ROOR), and hypochlorite (ClO−). In addition to ROS, other compounds involved in cellular redox homeostasis and signaling are the so-called reactive nitrogen species (RNS). These include nitric oxide or nitrogen monoxide (NO•), nitrogen dioxide (NO2•), peroxynitrite (ONOO−), alkyl peroxynitrite (ROONO), and nitroxyl anion (NO−), among others, all originating from NO•, which in turn is mainly produced by nitric oxide synthase enzymes, being predominantly a chemical messenger rather than a harmful species under physiologic conditions.
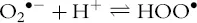
The superoxide radical anion (O2•−) is the prevailing form of superoxide in water at neutral pH (Eq. 1.2). It is a relatively persistent radical species, with limited reactivity toward biomolecules and a modest oxidizing character. Indeed, the standard redox potential of the redox couple O2/O2•− (−0.3 V vs. SHE at pH 7.0) suggests that it can, instead, reduce free and most chelated Fe3+ (e.g., Fe3+-citrate, Fe3+-ADP or Fe3+-cytochrome c) to the ferrous (Fe2+) species. Its modest reactivity is paradoxically the main reason for its importance in oxidative stress, as it allows this species to diffuse at relatively long distance from the site of origin and act as a chemical messenger, influencing a multitude of redox-regulated processes. Conversely, its neutral form (HOO•), which may predominate at lower pH (pKa = 4.8) or locally, in the proximity of a carboxylic group (–COOH, e.g., in proteins), possesses a far higher reactivity and oxidizing ability, similar to peroxyl radicals.
At the opposite end of the reactivity scale, among the radical species found in biological systems, HO• and RO• radicals have largely unselective behavior, being able to attack almost any biomolecule found in the proximity of their site of generation. These species, formed predominantly by Fenton-type chemistry (Eq. 1.2), by radiolysis of water, or by photolysis of peroxides and hydroperoxides, commonly react by H-atom abstraction from a C–H moiety (e.g., from lipids) or by addition to CӒC double bonds. The resulting carbon-centered radicals, under aerobic conditions, will react at near-diffusion controlled rates with oxygen to form a peroxyl radical, the main protagonis of oxidative stress. Peroxyl radicals are electron-poor highly reactive species that, more often, attack biomolecules by H-atom abstraction from –OH, –SH, and –CH functions. Unlike HO•, they are quite selective and attack only specific molecular sites.
1.2 Oxidative Damage to Biomolecules
1.2.1 Lipid peroxidation
The main ROS-related damage to lipids is lipid peroxidation, a radical-chain reaction mediated by peroxyl radicals. Several radical species including HO•, HOO•, RO•, and ROO• can act as initiating species by attacking unsaturated fatty acid residues and abstracting a hydrogen atom in the allylic (or bis-allylic) position to yield the corresponding alkyl (C-centered) radical, which ...
Inhaltsverzeichnis
- Cover image
- Title page
- Table of Contents
- Front Matter
- Copyright
- Preface: Aging Bioactive Foods
- Contributors
- Chapter 1. Antioxidant Supplementation in Health Promotion and Modulation of Aging: An Overview
- Chapter 2. Dietary Effects on Epigenetics with Aging
- Chapter 3. Bioactive Foods in Aging: The Role in Cancer Prevention and Treatment
- Chapter 4. Vitamins and Older Adults
- Chapter 5. Food and Longevity Genes
- Chapter 6. Diet, Social Inequalities, and Physical Capability in Older People
- Chapter 7. Dietary Patterns/Diet and Health of Adults in Economically Developing Countries
- Chapter 8. Diet and Aging: Role in Prevention of Muscle Mass Loss
- Chapter 9. Dietary Calories on Cardiovascular Function in Older Adults
- Chapter 10. Mediterranean Lifestyle and Diet: Deconstructing Mechanisms of Health Benefits
- Chapter 11. Creatine and Resistance Exercise: A Possible Role in the Prevention of Muscle Loss with Aging
- Chapter 12. Exercise in the Maintenance of Muscle Mass: Effects of Exercise Training on Skeletal Muscle Apoptosis
- Chapter 13. Taurine and Longevity – Preventive Effect of Taurine on Metabolic Syndrome
- Chapter 14. Preventing the Epidemic of Mental Ill Health: An Overview
- Chapter 15. Energy Metabolism and Diet: Effects on Healthspan
- Chapter 16. Nutritional Hormetins and Aging
- Chapter 17. The Health Benefits of the Ayurvedic Anti-Aging Drugs (Rasayanas): An Evidence-Based Revisit
- Chapter 18. Selenium, Selenoproteins, and Age-Related Disorders
- Chapter 19. Antioxidants and Aging: From Theory to Prevention
- Chapter 20. Diet and Brain Aging: Effects on Cell and Mitochondrial Function and Structure
- Chapter 21. Bioactive Prairie Plants and Aging Adults: Role in Health and Disease
- Chapter 22. Ginseng and Micronutrients for Vitality and Cognition
- Chapter 23. Asian Medicinal Remedies for Alleviating Aging Effects
- Chapter 24. Legumes, Genome Maintenance, and Optimal Health
- Chapter 25. Minerals and Older Adults
- Chapter 26. Nutritional Influences on Bone Health and Overview of Methods
- Chapter 27. Skeletal Impact of Soy Protein and Soy Isoflavones in Humans
- Chapter 28. Soy: Animal Studies, Spanning the Lifespan
- Chapter 29. Skeletal Effects of Plant Products Other Than Soy
- Chapter 30. Molecular Mechanisms Underlying the Actions of Dietary Factors on the Skeleton
- Chapter 31. Aging, Zinc, and Bone Health
- Chapter 32. General Beneficial Effects of Pongamia pinnata (L.) Pierre on Health
- Chapter 33. Nutrition, Aging, and Sirtuin 1
- Chapter 34. Inhibitory Effect of Food Compounds on Autoimmune Disease
- Index
Zitierstile für Bioactive Food as Dietary Interventions for the Aging Population
APA 6 Citation
Watson, R. R., & Preedy, V. (2012). Bioactive Food as Dietary Interventions for the Aging Population ([edition unavailable]). Elsevier Science. Retrieved from https://www.perlego.com/book/1835240/bioactive-food-as-dietary-interventions-for-the-aging-population-bioactive-foods-in-chronic-disease-states-pdf (Original work published 2012)
Chicago Citation
Watson, Ronald Ross, and Victor Preedy. (2012) 2012. Bioactive Food as Dietary Interventions for the Aging Population. [Edition unavailable]. Elsevier Science. https://www.perlego.com/book/1835240/bioactive-food-as-dietary-interventions-for-the-aging-population-bioactive-foods-in-chronic-disease-states-pdf.
Harvard Citation
Watson, R. R. and Preedy, V. (2012) Bioactive Food as Dietary Interventions for the Aging Population. [edition unavailable]. Elsevier Science. Available at: https://www.perlego.com/book/1835240/bioactive-food-as-dietary-interventions-for-the-aging-population-bioactive-foods-in-chronic-disease-states-pdf (Accessed: 15 October 2022).
MLA 7 Citation
Watson, Ronald Ross, and Victor Preedy. Bioactive Food as Dietary Interventions for the Aging Population. [edition unavailable]. Elsevier Science, 2012. Web. 15 Oct. 2022.