
eBook - ePub
Guide to Preparing the Corporate Quality Manual
Bernard Froman
This is a test
- 232 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Guide to Preparing the Corporate Quality Manual
Bernard Froman
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
Utilizes advanced concepts, guidelines and requirements from the latest ISO 9000 and 10000 series of standards, as well as other models, including TQM (Total Quality Managment). The text shows how to define a policy and explain it clearly. It offers procedures for developing a quality manual, to be used by personnel performing quality-related functions and for external auditors and customers.
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Guide to Preparing the Corporate Quality Manual als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Guide to Preparing the Corporate Quality Manual von Bernard Froman im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Negocios y empresa & Operaciones. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
PART ONE - GENERAL PRINCIPLES
1 The Concepts of Quality Management and Quality Assurance
During the last few years, quality concepts have evolved a great deal (see introduction); therefore, it is important, before working on the preparation of the quality manual, that within the context of an organization's quality approach everyone agree on well-defined concepts and terminology so that users of the manual may understand clearly its content. It is best to refer to the most recent definitions as these have been the object of international consensus.
The principal concepts and the terminology currently retained by the international and French standards are contained in the ISO 8402 standard, the 1994 revision of which is translated into the European and French NF EN ISO 8402. The NF X 50-125 French standard, which contains additions to ISO 8402, will replace the French NF X 50-120 standard.
1.1 Quality
Quality is the "totality of characteristics of an entity that bear on its ability to meet stated and implied needs" (see ISO 8402).
In this revised standard, the word "entity" refers not only to a product or service as in the previous definition, but also to an activity, a process, an organization or a person. The word "product," considered as the result of the activities and processes, can also refer to raw materials, hardware, software, services, etc.
The satisfaction of the various needs (see Introduction) implies that quality is the goal set throughout the industrial process (in the case of a product), or tertiary process (commerce or administration) or throughout the life cycle of the product: design, production, maintenance, etc. (see lower part of figure 1.1).
The introduction of the revised ISO 8402 standard broadly explains the quality concept and its different aspects.
1.2 Quality control and quality inspection
Quality control is defined as follows: "Operational techniques and activities that are used to fulfill requirements for quality" (see ISO 8402). These can be operational actions that can be applied to a pilot process (manufacturing progress, successive stages of a service) or to the elimination of nonconformities or deviations throughout a process in order to achieve the results expected.
Inspection is a quality control operation undertaken at a given stage of the process in question, to determine if, at that stage, the results obtained are in accordance with the specified requirements.
The quality control operations are the concern of the operational hierarchy, which has the responsibility for obtaining quality throughout this process (see figure 1.1).
1.3 Quality assurance
Quality assurance (often abbreviated as "QA ") refers to "ali the planned and systematic activities implemented within the 'quality systern' (see ISO 8402) and, demonstrated as needed, to provide adequate confidence that an entity will fulfill requirements for quality" (see ISO 8402). Quality assurance consists of:
- - A coordinated group effort aimed at:
- building confidence inside the organization that quality is being obtained.
- giving the customer (or regulatory authorities) confidence that quality is being obtained.
- - Planned and systematic actions (provided for within a quality system environment) aimed at an organization's systematically stating in a document (such as a quality plan) the "control" operations that will be necessary to obtain quality.
- - Effective demonstration of implemented actions through planned methods (documentation, quality audits, etc.). This demonstration must be based upon " objective evidence," and the " degree of demonstration " (see ISO 8402) is the extent to which evidence is produced, as needed, depending on technical complexity, safety etc. and cost criteria.
- - External auditing of the organization's QA program. As a precondition, it is necessary to establish confidence within the organization via the organization's internal QA (implying internal audits); this must be one of the main objectives of the quality assurance manual (or quality manual, if it is assuming a dual role).
Although some quality control and quality assurance actions are interrelated, there must be no confusion between the two concepts:
- - quality control relates to the fulfillment of quality requirements (operational or technical aspect) and,
- - quality assurance aims at providing confidence that the requirements are fulfilled, within the organization (internal QA) and outside it to satisfy customers or regulatory and legislative authorities (external QA).
Therefore, in a quality system, a procedure can be a quality control document, because of the technical requirements that it contains, as well as a quality assurance document that will provide confi
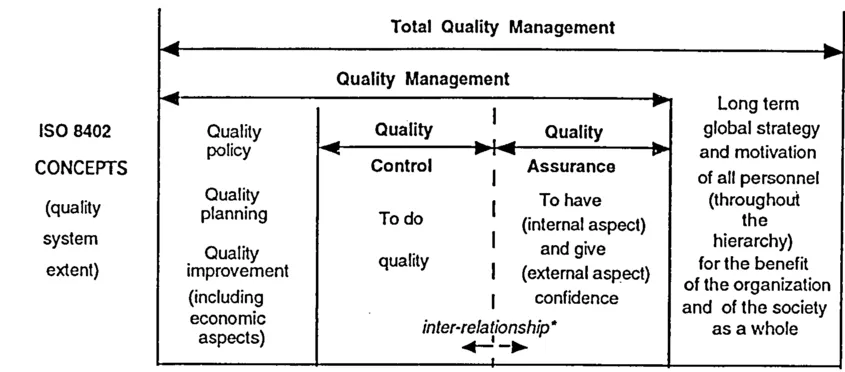
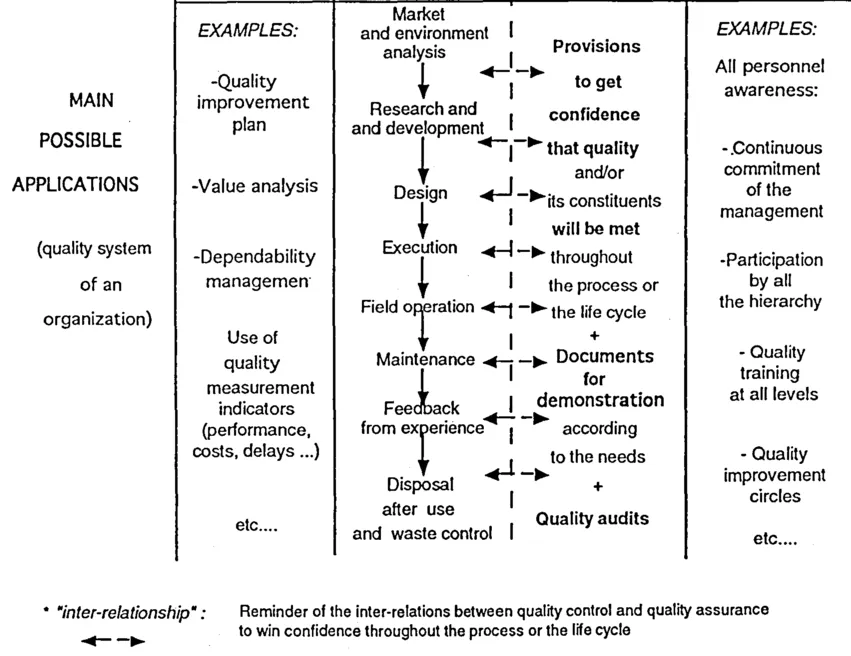
Figure 1.1 Constituents of quality management concepts
dence because of the complementary requirements it puts forth, which are aimed at demonstrating how quality will be achieved.
Quality assurance actions are generally carried out throughout a process, in a functional line, distinct from the quality control operational line, to meet the required independence that is necessary for securing confidence (see figure 1.1).
1.4 Quality management
Quality management is "all the activities of the overall management function that determine the quality policy, objectives and responsibilities and implement them by such means as quality planning, quality control, quality assurance and quality improvement, within the quality system" (see ISO 8402).
The way that management regards these aspects of quality reflects an organization's dedication to quality. Quality management consists, on one hand, of quality control and quality assurance and, on the other, of additional quality policy concepts such as quality improvement (including cost aspects). Quality management is applied to all the stages of a process or life cycle of a product or service; it can be extended to all the sections of an organization. It is implemented within or by an organization by setting up a quality system.
The quality system is, by definition, the "organizational structure, procedures, processes and resources needed to implement quality management" (see ISO 8402). It comprises therefore all the quality-related provisions that can be applied in full or in part by an organization and also throughout the life cycle of a product. In this sense, it can also be said that quality management "operates throughout the quality system" (see ISO 8402, Introduction).
Note that the term "quality assurance system," which is generally not an accepted meaning or definition, is to be avoided.
Total Quality Management (or TQM) is an extension of the quality management concept, as it pertains to the participation and motivation of all the members of the organization (from the top to the bottom of the hierarchy) in its own interest and for the benefit of its environment. Depending on the areas and on the different cultures, this concept can have various forms and designations. For example, total quality, "companywide quality control" (CWQC), "management by total quality," or even designations of certain quality awards (Malcolm Baldrige, European Award (EFQM), French Quality Award). This term and its definition have been the object of an international consensus reported in the ISO 8402 standard (see § 3.7 and especially the corresponding note 5).
The relationship between these concepts and their possible application for an organization's quality system are shown in figure 1.1. Note that:
- - the vertical arrows in the quality control column, which illustrate a series of elements in the "quality system" that can be the object of quality control and assurance provisions throughout the life cycle of a product (some are the object of standards - see ISO 9001 paragraph 4.4 for design, for example; others are not, or not yet, for example, field operation; certain organizations may find it useful or necessary to deal with these elements in their quality manuals);
- - the horizontal "inter-relationship" arrows, which refer to the links between quality control and quality assurance throughout the process or life-cycle (to obtain confidence throughout the quality system).
For clarity's sake, these concepts are summarized in figure 1.2
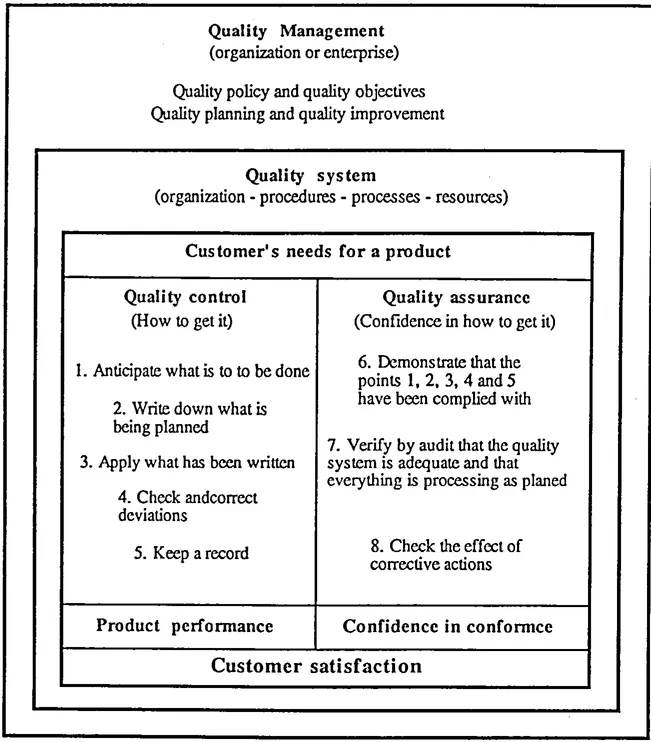
Figure 1.2 Simplified pedagogical approach to the quality management concepts
2 The Quality Manual Within the Series of Quality Systems Documents
2.1 The quality documents pyramid
The documents relating to the "quality system" of an organization, i.e. relating to "organizational structure, procedures, processes and resources needed to implement quality management" (see ISO 8402), are:
- - the quality (or quality assurance) manuals,
- - the quality (or quality assurance) plans,
- - the procedures,
- - the various operational documents for the implementation of the quality system: instructions, operating methods management notes, specifications, forms, manufacturing and inspection process sheets, quality audit programs, minutes, certificates, reports, etc., and, more generally, all the records demonstrating how quality is obtained.
The nature of these documents, their importance in numbers, their typology and their user guide, depend of course on the nature of the organization (industrial group, small/medium sized enterprises/industries, production plant, service company, administration, etc.).
Quality (or QA) manuals are often represented at the top of the pyramid of the various quality documents because, by describing the organization's entire quality system in a very general manner, they refer to the overall existing quality implementation documents that they cover, i.e. the general procedures, or documents specific to various operations.
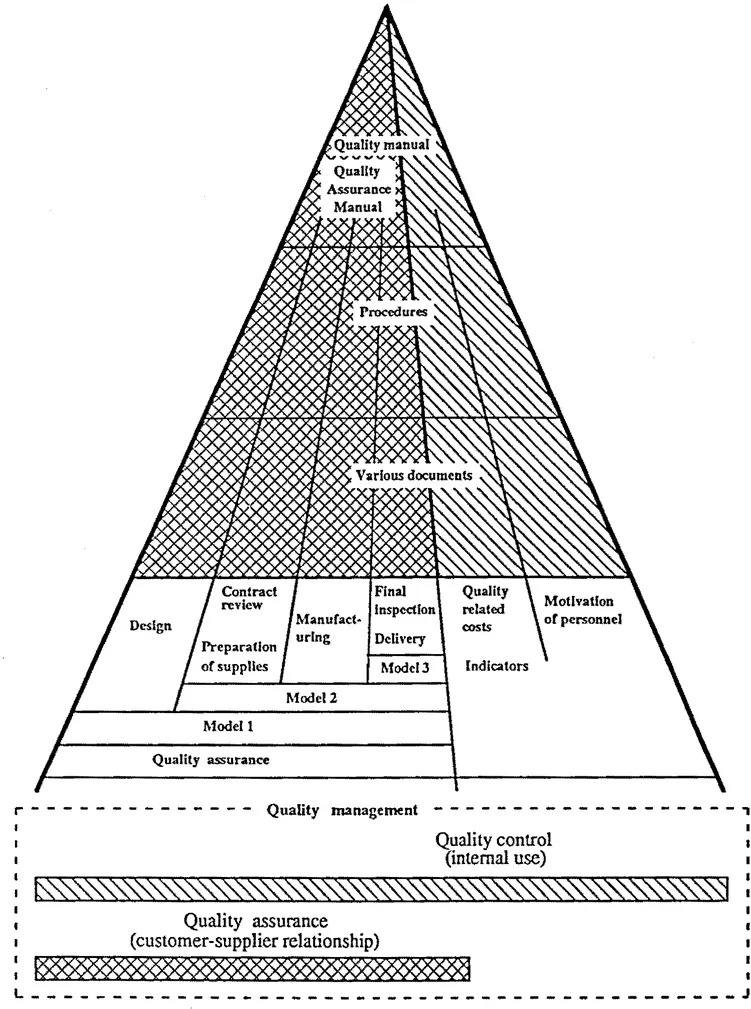
Figure 2.1 Quality documents pyramid
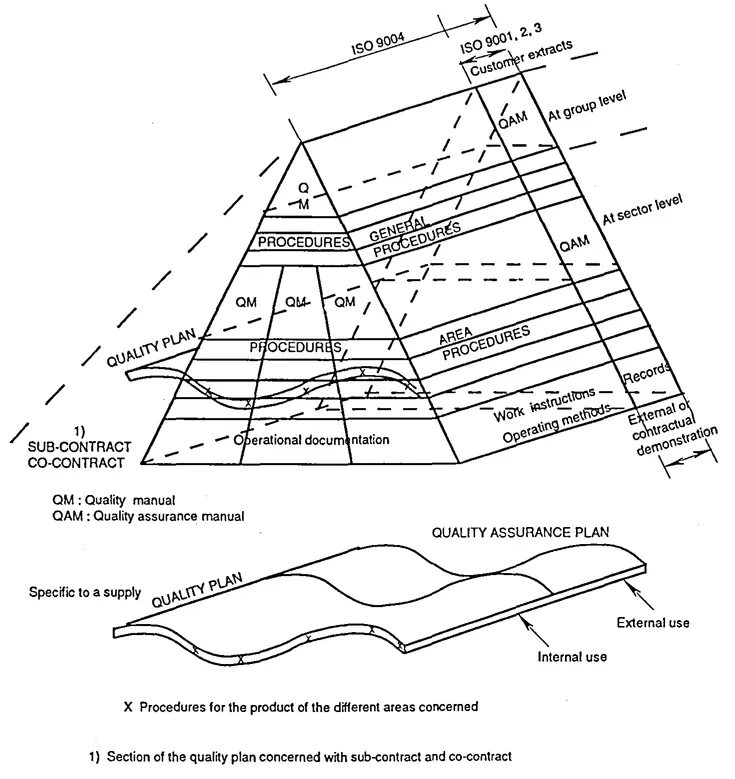
Figure 2.2 Example of breakdown and application of quality documents
The pyramid in figure 2.1 emphasizes the di...
Inhaltsverzeichnis
- Cover
- Half Title
- Series Page
- Title Page
- Copyright Page
- About the Series
- Foreword
- Preface
- Contents
- List of Figures
- INTRODUCTION
- PART ONE - GENERAL PRINCIPLES
- PART TWO - CONTENTS OF A QUALITY MANUAL
- CONCLUSION
- NORMATIVE DOCUMENTS
- INDEX
Zitierstile für Guide to Preparing the Corporate Quality Manual
APA 6 Citation
Froman, B. (2020). Guide to Preparing the Corporate Quality Manual (1st ed.). CRC Press. Retrieved from https://www.perlego.com/book/2014111/guide-to-preparing-the-corporate-quality-manual-pdf (Original work published 2020)
Chicago Citation
Froman, Bernard. (2020) 2020. Guide to Preparing the Corporate Quality Manual. 1st ed. CRC Press. https://www.perlego.com/book/2014111/guide-to-preparing-the-corporate-quality-manual-pdf.
Harvard Citation
Froman, B. (2020) Guide to Preparing the Corporate Quality Manual. 1st edn. CRC Press. Available at: https://www.perlego.com/book/2014111/guide-to-preparing-the-corporate-quality-manual-pdf (Accessed: 15 October 2022).
MLA 7 Citation
Froman, Bernard. Guide to Preparing the Corporate Quality Manual. 1st ed. CRC Press, 2020. Web. 15 Oct. 2022.