![]()
1
Introduction
The word pyrolysis, translated from the original Greek pyros = fire and lyso = decomposition, means a chemical transformation of a sample when heated at a temperature higher than ambient in an inert atmosphere in the absence of oxygen [1]. Pyrolysis can be divided into two types, applied pyrolysis and analytical pyrolysis [2]. Applied pyrolysis is concerned with the production of chemicals. When performed on a large scale, pyrolysis is involved in industrial processes as the manufacture of coke from coal and the conversion of biomass into biofuels. In contrast, analytical pyrolysis is a laboratory procedure in which small amounts of organic materials undergo thermal treatment. The pyrolysis itself is just a process that allows the transformation of the sample into other compounds. The pyrolytic process is carried out in a pyrolysis unit (pyrolyzer) interfaced with the analytical instrumentation.
1.1.Definition of the Analytical Pyrolysis
According to the International Union of Pure and Applied Chemistry (IUPAC) recommendation, analytical pyrolysis is defined as the characterization in an inert atmosphere of a material or a chemical process by a chemical degradation reaction(s) induced by thermal energy [3]. Thermal degradation under controlled conditions is often used as a part of an analytical procedure, either to render a sample into a suitable form for subsequent analysis by gas chromatography (GC), mass spectrometry (MS), gas chromatography coupled with the mass spectrometry (GC/MS), with the Fourier-transform infrared spectroscopy (GC/FTIR), or by direct monitoring as an analytical technique in its own right [3]. Analytical pyrolysis deals with the structural identification and quantitation of pyrolysis products with the ultimate aim of establishing the identity of the original material and the mechanisms of its thermal decomposition [4]. The pyrolysis temperatures of 550–1400°C are high enough to actually break molecular bonds in the molecules of the solid sample, thereby forming smaller, simpler volatile compounds. Depending on the amount of energy supplied, the bonds in each molecule break in a predictable manner [5]. Most of the degradation results from free radical reactions initiated by bond breaking and depends on the relative strengths of the bonds that hold the molecules together. A large molecule will break apart and rearrange in a characteristic way. If the energy transfer to the sample is controlled by temperature, heating rate, and time, the fragmentation pattern is reproducible and characteristic for the original polymer [6]. Another sample of the same composition heated at the same rate to the same temperature, for the same period of time, will produce the same fragments. By the identification and measurement of the fragments, the molecular composition of the original sample can often be reconstructed. Pyrolysis may not be thought of as a sample preparation technique, but more of a sample destructive technique since the actual molecular form of the sample is changed upon heating. The chronological development of the analytical pyrolysis is described in detail in the publication of Tsuge [7].
1.2.Application of the Analytical Pyrolysis
Pyrolysis is often used for the analysis of polymer and copolymer samples since they are too high of a molecular weight (MW) to analyze using GC techniques and they often degrade in a systematic fashion. The applications of the analytical pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS) range from research and development of new materials, quality control, characterization and competitor product evaluation, medicine, biology and biotechnology, geology, airspace, environmental analysis to forensic purposes, or conservation and restoration of cultural heritage. These applications cover analysis and identification of polymers/copolymers and additives in components of automobiles, tires, packaging materials, textile fibers, coatings, adhesives, half-finished products for electronics, paints or varnishes, lacquers, leather, paper or wood products, food, pharmaceuticals, surfactants, and fragrances [8].
1.2.1.Analytical pyrolysis of synthetic organic polymers and copolymers
The word polymer, translated from original Greek, means many parts. Structural analysis and the study of the degradation properties of high-MW polymers, such as plastics, rubber, and resins, are important to understand and improve performance characteristics of a polymer in many industrial applications. Polymers cannot be analyzed in their normal state by traditional GC because of their high MW and lack of volatility.
Although synthetic polymers and copolymers are inherently difficult to analyze because of their high MW and lack of volatility, various analytical techniques are used to characterize polymers/copolymers. These techniques including physical testing (rheological testing), thermogravimetric analysis (TGA), electron microscopy, Fourier transform infrared (FTIR) spectroscopy, size-exclusion chromatography (SEC)/gel permeation chromatography (GPC), nuclear magnetic resonance (NMR), laser light scattering, ultraviolet–visible (UV/Vis) spectroscopy and MS. The listed nondestructive methods offer information about functional groups, structural elements, thermal stability, MW, and volatile components. They have limitations and often laborious and time-consuming sample preparation, including hydrolysis, dissolution, or derivatization, is needed before analysis. Analytical pyrolysis (Py) technique hyphenated to GC/MS has extended the range of possible tools for the characterization of synthetic polymers and copolymers. This technique has been used extensively over the last 30 years as a complementary analytical tool used to characterize the structure of synthetic organic polymers and copolymers, polymer blends, biopolymers, and natural resins. Pyrolysis–GC/MS is a destructive analytical technique. Typical fields of interest and application are [9–12]:
—polymer identification by comparison of pyrograms and mass spectra with known references;
—qualitative analysis and structural characterization of copolymers, sequence statistics of copolymers, differentiation between statistical and block polymers;
—determination of the (micro) structure of polymers (degree of branching and crosslinking, compositional analysis of copolymers and blends, co-monomer ratios, sequence distributions, analysis of end-groups);
—determination of the polymers steric structure (stereoregularity, tacticity, steric block length, and chemical inversions);
—investigation of thermal stability, degradation kinetics, and oxidative thermal decomposition of polymers and copolymers;
—determination of monomers, volatile organic compounds (VOCs), solvents, and additives in polymers;
—kinetic studies;
—quality control; and
—quantification.
![]()
2
Apparatus in Analytical Pyrolysis
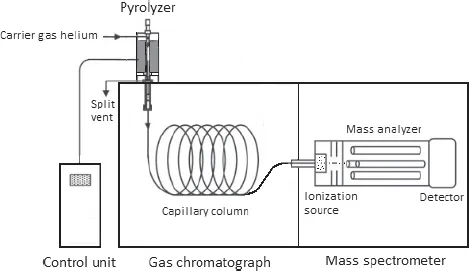
Figure 2.1 illustrates a schematic diagram of a pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS) measuring system with the direct connections of an oven pyrolyzer together with the temperature and pressure controller, a gas chromatograph (GC) equipped with a capillary separation column, and detection device using a quadrupole mass spectrometer (MS).
Pyrolysis method allows for the direct analysis of very small solid or liquid polymer/copolymer sample amounts (5–200 µg) and eliminates the need for time-consuming sample preparation (pre-treatment) by performing analyses directly on the sample. When a polymer/copolymer sample is subjected to pyrolysis, primary bond-fission processes are initiated. These may proceed by means of several temperature-dependent and competing reactions, which make the final fragment distribution highly dependent upon the pyrolysis temperature. Therefore, the essential requirements for the apparatus in analytical pyrolysis are reproducibility of the final pyrolysis temperature, rapid temperature rise, and accurate temperature control. Depending upon the heating mechanism, pyrolysis systems have been classified into two groups: the continuous-mode pyrolyzer (furnace pyrolyzer) and pulse-mode pyrolyzer (flash pyrolyzer), such as the heated filament, Curie-point, and laser pyrolyzer. The pyrolysis unit is connected directly to the injector port of a GC (Figs. 2.2 and 2.3).
Fig. 2.1. Schematic diagram of a Py–GC/MS apparatus (based on Ref. [6]).
Fig. 2.2. Furnace pyrolyzer Pyrojector IITM (SGE Analytical Science, Melbourne, Australia) used in this work connected to a 7890A GC and a series 5975C quadrupole MS (Agilent Technologies Inc., Santa Clara, CA, USA).
Fig. 2.3. Furnace pyrolyzer Pyrojector IITM (SGE Analytical Science, Melbourne, Australia) used in this work connected to a Trace 2000 GC (ThermoQuest CE Instruments, Milan, Italy) and a Voyager quadrupole MS (ThermoQuest/Finnugan MassLab Group, Manchester, UK).
Once the polymer/copolymer sample has been pyrolyzed, volatile fragments are swept from the heated pyrolysis unit by the carrier gas (helium) into the GC. The volatile pyrolysis products (pyrolyzate) are chromatographically separated by using a fused silica capillary column according to the boiling points and the affinity of analytes to the stationary phase (internal capillary column wall coating). The detection technique of the separated compounds is typically MS, but other GC detectors have also been used depending on the intentions of the analysis. The substances detected by the MS are subsequently identified by the interpretation of the obtained mass spectra, by using mass spectra libraries (e.g. NIST/EPA/NIH, Wiley, MPW, Norman Mass Bank, m/z Cloud), or by using reference substances. The identification of complex mixtures or blends as well as identification of samples with so-called “difficult matrices” is also possible in many cases. Due to these small sample amounts, the investigation of heterogeneous polymers with a coarse phase or a gradient composition structure (phase separation, poor mixing, etc.) is sophisticated and may lead to great variations in the measuring results. In this case, a multiple determination of different positions of the investigated part is essential to achieve a significant image of its composition [12].
2.1.Furnace Pyrolyzer
T...