![]()
Part 1
The Submicroscopic World
ENRICO FERMI (1901 – 1954)
“The total number of electrons, as well as neutrinos, is not necessarily constant. Electrons (or neutrinos) can be created and annihilated.”
![]()
Elementary particles
Today is the particle era in physics. The downward probing of the past few centuries has led finally in the last few decades to the world of elementary particles. Actually no scientist believes that all the particles we now know are truly elementary, but for lack of a better word, and more especially for lack of any deeper knowledge of a lower substratum of matter, they bear the name elementary. It is the effort to understand the particles, to tie them together somehow through deeper and simpler concepts, that occupies the attention of many scientists throughout the world.
2.1The submicroscopic frontier
8
Why do we begin a study of physics by examining the modern microscopic frontier represented by the elementary particles? In part it is to give a view of one of the furthest points of advance of physical science in order to provide a reference point as the theories of physics are developed, approximately in historical order, in the subsequent parts of the book. There is about the particles also the paradoxical fact that, although their discovery rested upon all the earlier developments of science, they are themselves rather easily visualizable and some of their main properties can be understood with no background of science at all. We can think of a golf ball or a marble, and extrapolating downward many orders of magnitude, picture an elementary particle as a tiny speck of matter, a basic building block of the universe. Of course, some of the properties of the particles, especially the way in which they interact with one another, require for their understanding a knowledge of some or all of the main theories of physics. These aspects of the particles are saved for appropriate places in later chapters.
The modern scientist, whether biologist, chemist, or physicist, is decidedly microscopically oriented. He pictures the large-scale world as put together from smaller and smaller units, down to the elementary particles and someday soon, perhaps, to a still deeper unit. And he pictures events and phenomena as arising from laws which ultimately have their simplest expression in the world of the very small. If Aristotelian physics was a science of final causes, modern physics is a science of microscopic causes. “Explanation” in physical science is, as often as not, the description of something larger in terms of something smaller. In looking first at the elementary particles, we are examining the basis of physical science as well as its frontier.
The elementary particles are useful objects for illustrating many aspects of physics. Their very simplicity means they are better adapted for illustrating basic principles of science than are the unwieldy and more complicated objects of our everyday life. We shall see in Chapter Four how the elementary particles illustrate the all-important conservation laws. Throughout the remainder of the book, we shall find the particles convenient for demonstrating aspects of each of the great theories of physics.
About the particles we now know a great deal, but not nearly enough. They remain very puzzling. Looking first at the particles will emphasize, as eighteenth-and nineteenthcentury science could not do nearly so well, the exploratory and tentative nature of science. With the particles, the known and the unknown appear equally exciting.
To the scientist, the elementary particles provide the greatest challenge in modern physics. To the student they provide important insight into the scientist’s view of the world. It is well to recall that the most familiar points of impact of physical science on man—our satellite communications, our detergents, our bombs, our household gadgets—form only a sideline off the mainstream of scientific advance. The true frontiers of science lie far more remote from everyday life, and one of these is the submicroscopic frontier inhabited by the particles. Being pursued by the most refined experimental and mathematical techniques, the particles may soon give way and allow the frontier to be extended to a still smaller domain.
2.2The early particles
Man and the familiar objects of his world are constructed from atoms and molecules. At the turn of this century, atoms were known to exist but, as with today’s elementary particles, the structure of the atom and the relation of one atom to another were mysteries. It was known that the atom was the smallest unit of an element such as hydrogen or oxygen or sodium or uranium; that there were somewhat over eighty different kinds of atoms (today we know just over a hundred); and that atoms were all of about the same size, such that one hundred million of them side by side would stretch into a line less than one inch long. It was also known that groups of different kinds of atoms could join together to form tiny structures called molecules, which, in turn, became the basic building blocks of the vast and wonderful variety of substances we encounter in the world.
In the first decade of the twentieth century discoveries came thick and fast, leading to a giant step downward toward the subatomic world of particles. The electron, the first known particle, had been discovered in 1897 by J. J. Thomson in England. He showed that the “cathode rays” produced by high voltage applied within an evacuated vessel could be deflected both electrically and magnetically. The rays were most simply interpreted as a beam of high-speed identical particles carrying negative electric charge (the same sign as the charge on an electrified rubber balloon). That the mass of each of these particles was considerably less than the mass of a single atom was verified by the ease of deflecting them. That each also had a size much less than the size of an atom was shown by their power to penetrate through a gas. Thomson realized, as those who had studied cathode rays before him had not, that he was dealing with truly subatomic objects, even though he was still far from isolating a single one of them.
The electron, as soon as it was identified, was strongly suspected to be a particle contained within atoms. As a common constituent of atoms, it provided the first definite link between distinct atoms. Another important link was forged when, in 1902, it was discovered that a radioactive atom (one capable of spontaneously emitting powerful radiation) could transmute itself into an entirely different kind of atom. This transmutation strongly suggested that atoms must be not independent, indivisible entities, but structures built up of some common, more elementary building blocks.
The alpha particles ejected at high speed from radioactive atoms were used shortly thereafter as the first projectiles for bombarding atoms. (These atomic “bullets,” conveniently provided free by nature, are not energetic enough for modern purposes; today they are replaced by particles artificially pushed to still higher speeds in giant accelerators.) A result of the early bombardments was the revelation that the interior of atoms is largely empty space. By 1911 the experimenter Ernest Rutherford had discovered that the atom contained a massive, positively charged core—the nucleus—at least ten thousand times smaller than the atom as a whole, and that the remaining space was occupied by a few light-weight, negatively charged electrons. Two years later the theorist Niels Bohr provided a successful mathematical description of the motion of the electrons in the atom. Despite later modifications of detail, this description remains, in its essentials, our picture of atomic structure up to the present. The electrons whirl rapidly about the nucleus, providing a sort of atomic skin just as a whirling propeller seems to form a disk. Like a bullet that passed safely between the propeller blades of a World War I fighter plane, a high-speed particle can readily penetrate the electron cloud to reach deep within the atom. Another atom, however, drifting up with a relatively low speed, is turned back at the periphery, much as a stone tossed slowly at a spinning propeller might be batted back.
The nucleus of the lightest atom, hydrogen, was christened the proton and joined the electron to bring the list of elementary particles of fifty years ago up to two. Heavier nuclei were believed to be formed of a number of protons and electrons packed closely together. The details of this picture of nuclear structure were never clear; indeed, it was abandoned in 1932. However, it was a most attractive idea to have just two fundamental particles—the negatively charged electron and the positively charged proton—from which all of the matter in the universe was constructed. The only puzzling feature (a puzzle not yet resolved) was why the proton should be much heavier than the electron. In any case, this idyllic twoparticle situation was not to last.
Several things happened in the early 1930s to begin the disturbing increase in the number of known elementary particles which has continued until the present time. A new particle, the neutron, was discovered; it was about as massive as the proton but carried no electric charge. The neutron was quite welcome, for it was just the particle needed to join the proton to form atomic nuclei. The picture of the nucleus immediately adopted and still accepted was that of a collection of protons and neutrons glued tightly together by a new strong force, simply called the nuclear force. For example, U235, the most famous isotope of uranium, has a nucleus consisting of 92 protons and 143 neutrons. The far simpler nucleus of helium (the same thing as an alpha particle*) contains two neutrons and two protons.
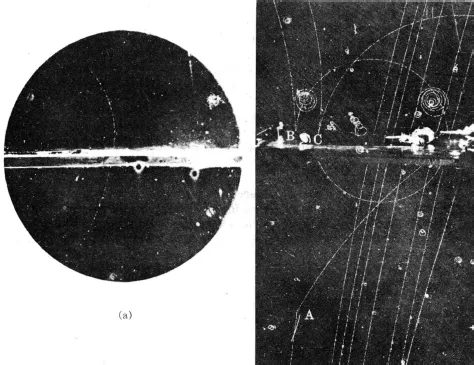
FIGURE 2.1 Positron tracks. (a) Carl Anderson’s photograph first served to identify the positron in 1932. Entering the cloud chamber at the bottom, the positron is deflected into a curved path by a magnetic force. After being slowed down in the central metal plate, it is more strongly deflected. An electron trajectory would have curved in the opposite direction. (b) This more recent bubble chamber photograph shows tracks of both positrons and electrons (as well as other particles). High-energy photons (gamma rays) emanating from point A create electron-positron pairs at points B and C. The positrons arc upward to the left. The electron tracks bend away to the right. [(a) Photograph courtesy of Carl D. Anderson, California Institute of Technology. (b) Photograph courtesy of Lawrence Radiation Laboratory, University of California, Berkeley.]
At nearly the same time, a fourth particle was discovered by the track it left in a cloud chamber exposed to cosmic radiation in Pasadena, California. This new particle, the positron, was as light as the electron but carried a positive instead of a negative charge. Some positron tracks are shown in Figure 2.1. Like the neutron, the positron arrived at an opportune time. A few years earlier, in 1928, Paul Dirac had constructed a new theory of the electron that was brilliantly successful in accounting for the fine details of atomic structure. But Dirac’s theory seemed to have one flaw. It predicted a sister particle for the electron, alike in all respects except the sign of its electric charge. A slot in the structure of theoretical physics was ready and waiting for the positron when Carl Anderson discovered it in 1932. (Dirac’s theory also predicted a negatively charged sister for the proton, called the antiproton, but many years had to go by before it was seen. The construction of the six-billion-volt Bevatron in Berkeley, California, made possible the production of antiprotons, and they were first observed in Berkeley in 1955.)
The advance of physical theory in the late 1920s was responsible for the “rediscovery” of an old particle, the photon. Back in 1905, the same year his first important paper on relativity was published, Albert Einstein had shown that a phenomenon called the photoelectric effect (which is discussed in Section 24.1) could best be understood with the assumption that light waves are absorbed only in bundles of a definite energy. These energy packets, now called photons, behaved in some ways like particles, yet were quite different from ordinary material particles. Although they carried energy, they had no mass. They could be neither speeded up nor slowed down, but traveled always at the same invariable (and enormous) speed. They could be born and die (that is, be emitted and absorbed), whereas ordinary particles—or so it was then believed—remained in existence forever. And, unlike material particles, the photon could never be isolated at a particular point except during the moment of its birth or death; otherwise it spread in a diffuse manner through space. For all of these reasons, the photon was not associated with the electron and the proton as a true elementary particle.
The theory of quantum mechanics, discovered in 1925 and developed over the next decade, changed this view of the photon. It showed that, from a fundamental point of view, the difference between a photon and a material particle was not so great. The particle happened to have mass; the photon did not. All of their other dissimilarities could simply be understood as arising from this single difference. In particular, the quantum theory suggested that it should be possible for material particles to be created and annihilated. The photon appeared not to be so distinctive after all and it joined the list of elementary particles.
9
Very shortly a theory developed by Enrico Fermi showed that man had, in fact, been witnessing th...