![]()
1
Quality by Design
Vince McCurdy
1.1 History
The pharmaceutical industry has been a highly regulated industry in the past for many good reasons [1]. While pharmaceuticals have greatly improved the mortality and morbidity rates, there is still some element of risk to the patients. These risks are greatly mitigated with the delivery of medicine at the appropriate purity, potency, delivery rate, and so on. While pharmaceutical regulations have clearly protected the population from much of the needless harm such as that incurred early in the twentieth century, there has been a concern more recently that overregulation may be associated with stifling innovation that can improve pharmaceutical quality even further [2] – innovation that has the potential to greatly improve the quality, cost, and time to market new and improved medicines. The twenty-first century began with the pharmaceutical industry using manufacturing technologies that have been employed since the 1940s and did not make significant changes in manufacturing process unless significant compliance or costs saving advantages could justify the high costs and long cycle time needed to gain approval. This often resulted in inefficient, overly expensive processes that were ultimately not in the best long-term interests of patients. As a result, the FDA (Food and Drug Administration) and other agencies around the world have embraced a new paradigm for regulation [3]. The “desired state” was to shift manufacturing from being empirical to being more science, engineering, and risk based. Another regulatory guidance that had major impact was the Process Analytical Technology (PAT) Guidance [9]. The continuous, real-time monitoring of manufacturing processes is a key enabler to achieve greater process control. Finally, the current Good Manufacturing Practices (cGMPs) for the Twenty-First Century Guidance acknowledged the undesired impact of good manufacturing practices (GMPs) on understanding manufacturing science and sought to set the framework for additional guidances that encouraged risk- and science-based understanding in exchange for more freedom to introduce innovations and improvements that will result in enhanced quality, cost, or timing.
Juran is often credited with introducing the concepts behind Quality by Design (QbD) [4]. Pharmaceutical QbD is a systematic approach to development that begins with pre-defined objectives and emphasizes product and process understanding based on sound science and quality risk management (ICH Q8R2). The holistic and systematic approach of QbD was relatively new to the pharmaceutical industry at the beginning of the twenty-first century. However, elements of QbD were certainly being applied across the industry long before then. QbD was put into practice in a big way with the advent of the FDA CMC pilot program in 2005. Nine companies participated in the program and eventually submitted regulatory filings based on a QbD framework [1, 2, 5, 6, 7]. Much was learned from these initial filings that help steer the industry and regulators toward a common vision for QbD. A comparison of the “current state” to the future “desired state” was succinctly summarized by Nasr in Table 1.1 [8].
Table 1.1 Comparison of the Current State to the Future Desired QbD State
| Pharmaceutical development | Empirical; typically univariate experiments | Systematic; multivariate experiments |
| Manufacturing process | Locked down; validation on three batches; focus on reproducibility | Adjustable within design space; continuous verification within design space; focus on control strategy |
| Process control | In-process testing for go/no-go; offline analysis | PAT utilized for feedback and feed forward in real time |
| Product specification | Primary means of quality control; based on batch data | Part of overall quality control strategy; based on product performance |
| Control strategy | Mainly by intermediate and end product testing | Risk-based; controls shifted upstream; real-time release |
| Lifecycle management | Reactive to problems and OOS; postapproval changes needed | Continual improvement enabled within design space |
A process is well understood when
- all the critical sources of variability are identified and explained;
- variability is managed by the process, and;
- product quality attributes can be accurately and reliably predicted over the design space established for materials used, process parameters, manufacturing, environmental, and other conditions [9].
Process understanding is the major goal of a QbD program. A complete list of characteristics of a successful QbD program is summarized in Table 1.2.
Table 1.2 The Characteristics of a Successful QbD Program
| Involves product design and process development |
| Risk-based, science based |
| Primary focus is patient safety and product efficacy |
| Business benefits are also drivers |
| Results in improved process understanding |
| Results in improved process capability/robustness |
| Systematic development |
| Holistic – applies to all aspects of development |
| Multivariate – interactions are modeled |
| Provides PAR, design space, or suitable equivalent |
| Requires a significant reduction in regulatory oversight postapproval |
1.2 Defining Product Design Requirements and Critical Quality Attributes
In order to design quality into a product, the requirements for the product design and performance must be well understood in the early design phase. In pharmaceuticals, these product requirements can be found in a Quality Target Product Profile (QTPP). The QTPP is derived from the desired labeling information for a new product. Pharmaceutical companies will use the desired labeling information to construct a target product profile that describes anticipated indications, contraindications, dosage form, dose, frequency, pharmacokinetics, and so on. The target product profile is then used to design the clinical trials, safety and ADME studies, as well as to design the drug product, that is, the QTPP.
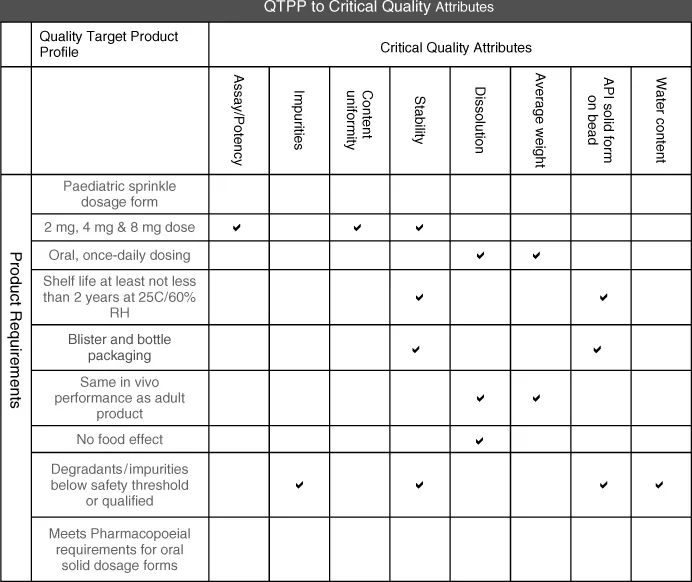
In addition to defining the requirements to design the product, the QTPP will help identify critical quality attributes such as potency, purity, bioavailability or pharmacokinetic profile, shelf-life, and sensory properties as shown in Figure 1.1. In some cases, these attributes are directly measurable, for example, potency. In other cases, surrogate measurements are developed indirectly to measure the quality or performance, for example, in vitro dissolution for a controlled release product.
There are numerous ways to represent a QTPP. Another example of a QTPP for a lyophilized sterile vial is shown in Table 1.3.
Table 1.3 Quality Target Product Profile for a Lyophilized Sterile Vial
| Indication | Chronic disease (treatment of nervous breakdown) |
| Dosage form | Lyophilisate for solution for injection |
| Dosage strength | Nominal dose 20 mg/vial |
| Administration route | Subcutaneous (0.8 ml) |
| Reconstitution time | Not more than 2 min |
| Solution for reconstitution | 1 ml 0.9% saline (provided by the pharmacy) |
| Packaging material drug product | 2R glass vial, rubber stopper, meets pharmacopoeial requirement for parenteral dosage form |
| Shelf life | Two yr 2–8 °C |
| Drug product quality requirement | Meets pharmacopoeial requirement for parenteral dosage form as well as product specific requirements |
| Stability during administration | Reconstituted solution is stable for 24 h at temperature ≤30 °C |
A crucial element of QbD is to ensure that the measurement systems being used are truly assessing the quality of the product or performance. Very often it is the case that attributes that have little to do with quality are measured, for example, dissolution test for an immediate release Biopharmaceutical Classification System (BCS) class I drug (high aqueous solubility and high permeability). Drugs of this type are rapidly and completely absorbed; therefore, a dissolution test provides little value from a quality control perspective. Quality attributes can sometimes be modeled on the basis of first principles or other multivariate analysis. Predictive models are extremely important components of QbD [10]. In the case of bioperformance, predictive statistical, mechanistic, and analytical tools are being applied, which can guide Active Pharmaceutical Ingredient (API) particle size selection, dissolution method design, and setting specifications [11].
While a QTPP is basic to QbD, additional product or process design requirements may need to be considered while designing the manufacturing process for a new API or drug product. In API route design, major decisions need to be made regarding which chemistry will yield a synthetic route that delivers high purity at an acceptable cost [12]. Likewise, a drug product formulation and process technology decision needs to be made that also delivers a drug product that conforms to the quality requirements at an acceptable cost. An understanding of the product (formulation) design is critical to product performance. A clear rationale for why excipient types, grades, and amounts are selected is part of the product understanding. An understanding of which material attributes contribute most to the excipient functionality is important to performance. Supplier specifications may be a poor indicator of excipient functionality in a dosage form and hence may not be critical material attributes. In some cases, it may be necessary to introduce additional testing on incoming materials that are more relevant to how the excipient impacts the dosage form performance [13]. Likewise, the solid form of the API nee...