![]()
Section II
Agent Effects
![]()
9
Mustard Vesicants
Rama Malaviya, Diane E. Heck, Robert P. Casillas, Jeffrey D. Laskin, and Debra L. Laskin
CONTENTS
9.1 Background
9.2 Mustards
9.2.1 Physical and Chemical Properties of Mustards
9.2.2 Toxicity of Mustards
9.2.3 Mechanisms of Mustard Toxicity
9.2.3.1 Cytotoxicity and Cell Death
9.2.3.2 Inflammatory Cells and Mediators
9.2.3.3 Oxidative Stress
9.3 Effects of Mustards on the Respiratory Tract
9.3.1 Human Exposure
9.3.1.1 Acute Effects
9.3.1.2 Long-Term Effects
9.3.2 Animal Studies
9.4 Potential Targets for Therapeutic Intervention
9.5 Conclusions
Acknowledgments
References
9.1 Background
Chemical warfare agents, readily produced from commonly available reagents, have been used in combat since World War I. The fatalities and casualties caused by these agents are a source of constant psychological terror. Extensive production and stockpiling by different countries has also proven hazardous to civilians due to accidental exposures (Dacre and Goldman, 1996; Davis and Aspera, 2001; Weibrecht et al., 2012). Although the Chemical Weapons Convention (CWC), an arms control treaty that bans the production, stockpiling, and use of chemical weapons, has mandated the demilitarization of current stockpiles, chemical warfare agents are being used in isolated incidents and remain a threat.
Vesicants, including sulfur mustard, are cytotoxic blistering agents that cause chemical burns when in contact with the body (Graef et al., 1948). Chemical casualties and incapacitating injuries to the lung, skin, and eyes during World War I were mainly due to sulfur mustard (Smith et al., 1995). Since then, there have been several incidents of mustard use, with the most recent being the Iran–Iraq war in the 1980s and Syria in attacks on insurgents and civilians in 2017 and 2018. The severity of injuries varies depending on the route of exposure, proximity to the source of mustard, and environmental conditions (Graham and Schoneboom, 2013). Both acute and long-term toxicity have been noted following exposure to the mustards. This chapter summarizes the toxic effects of mustard exposure on the lung, with a focus on mechanisms of injury and disease pathogenesis, and potential therapeutics.
9.2 Mustards
Sulfur mustard (2, 2¢-dichloroethyl sulfide) and nitrogen mustard (bis (2-chloroethyl) methylamine) are bifunctional alkylating agents. Although never used in combat, nitrogen mustard was developed for this purpose during World War II. It is mainly used for clinical applications as a cancer chemotherapeutic agent. However, as nitrogen mustard is closely related chemically to sulfur mustard and is potent in causing debilitating injury to target organs; it has been classified as a high-priority chemical threat agent (Anslow et al., 1947; Graef et al., 1948).
9.2.1 Physical and Chemical Properties of Mustards
Mustards are stable, pale yellow to dark brown, oily liquids with a mustard or garlicky odor. They readily mix with organic solvents but are poorly soluble (0.06–0.07% at 10–20°C) in water (Dacre and Goldman, 1996; Kehe et al., 2008). In aqueous or alkaline solutions, nucleophilic substitution of chlorine atoms by hydroxyl groups results in the formation of thiodiglycol (TDG) and thiodiglycol sulfoxide. Due to its easy absorption into food, porous surfaces, paints, and rubber objects, coupled with its poor volatility (900 mg/m3 at 25 °C), sulfur mustard liquid tends to persist, contaminating surfaces for extended periods (Dacre and Goldman, 1996; Rosenblatt et al., 1975). Moreover, mustard vapors are much denser than air and therefore, stick to the ground, saturating the terrain for long durations. In this regard, snow samples from regions in Norway were found to be contaminated even 2 weeks after mustard exposure (Johnsen and Blanch, 1984). In warmer climates, however, mustard can vaporize, saturating the air.
9.2.2 Toxicity of Mustards
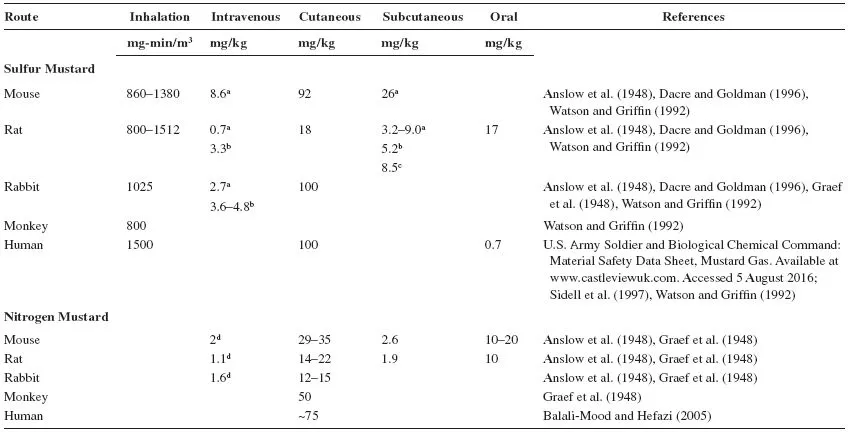
LD50 values for sulfur mustard and nitrogen mustard administered by different routes are presented in Table 9.1. It appears that in animals, the LD50 values are influenced by the solvent used to dissolve mustard (Anslow et al., 1947, 1948; Dacre and Goldman, 1996; Graef et al., 1948). Among the species tested, the lowest LD50 values have been observed in rats.
TABLE 9.1
LD50 of Mustards in Various Animal Species
Solvents used: aPropylene glycol; bneat; c95% alcohol; dsaline.
Mustards cause debilitating injury to the respiratory tract, eyes, and skin at low to moderate doses but are lethal at high doses (Anslow et al., 1947; Saladi et al., 2006; Smith et al., 1995). Systemic toxicity is also observed as a consequence of absorption into the circulation and distribution throughout the body (Dacre and Goldman, 1996). Depending on the dose and route of exposure, symptoms of injury may in some cases be delayed (Graef et al., 1948). Acute exposure to mustards is associated with salivation, lacrimation, respiratory-, gastric- and cardiac distress, and blistering of skin (Aasted et al., 1985; Anslow et al., 1948; Dacre and Goldman, 1996; Kehe et al., 2009). Neurologic disturbances including agitation, confusion, and ataxia have also been noted, along with conjunctivitis, headache, nausea, vomiting, diarrhea, and cachexia (Dacre and Goldman, 1996; Graef et al., 1948; Kehe et al., 2009). Injury to hematopoietic tissues results in leukopenia and immunodepletion, which in some cases leads to death due to infections (Alexander, 1947; Beattie and Howells, 1954; Kehe et al., 2009; Philips, 1950).
Studies on the clearance of mustard from the body suggest relatively slow excretion. In humans, non-hydrolyzed sulfur mustard (extracted and derivatized) has been detected in brain, fat, and skin, particularly in subcutaneous fat, even several days after exposure (Drasch et al., 1987; Kehe et al., 2008). The mustard metabolites thiodiglycol (TDG) and 1,1′-sulfonylbis [2-(methylthio)] ethane have been reported to persist in urine up to 10 days postexposure (Graham and Schoneboom, 2013). Additionally, blood protein adducts, although decreased in concentration over time, were detected after 40 days. Levels of TDG and protein adducts in blister fluid were 20 ng/ml and 68 pg/mg, respectively, 2 days postexposure, and these remained unchanged for up to 6 days (Graham and Schoneboom, 2013). Sulfur mustard itself has not been identified in blister fluid (Hurst et al., 2008).
9.2.3 Mechanisms of Mustard Toxicity
Due to its lipophilic nature, sulfur mustard rapidly penetrate tissues and cells. The initial reaction involves intramolecular cyclization to form an electrophilic ethylene sulfonium intermediate that can bind to a large number of biological molecules, including sulfhydryl, carboxyl, and aliphatic amino groups as well as heterocyclic nitrogen atoms (Giuliani et al., 1994; Kehe et al., 2008; Shakarjian et al., 2010; Smith et al., 1995, 1998). This results in alkylation and cross-linking of nucleic acids, proteins, lipids, and other membrane components (Papirmeister et al., 1991; Shakarjian et al., 2010). Since sulfur mustard is a bifunctional alkylating agent, it can form monofunctional, as well as intra- and intermolecular cross-links. Thus, DNA alkylation at the N7-position of guanine or the N3-position of adenine results in monofunctional adducts, whereas alkylation on two adjacent N7-positions of guanines on the same or on opposite strands of DNA, leads to the formation of intra- and intermolecular bifunctional adducts, respectively (Brookes and Lawley, 1961; Fidder et al., 1994; Kehe and Szinicz, 2005; Ludlum et al., 1994; Mol et al., 1993; Papirmeister et al., 1985). In mammalian cells, approximately 65% and 17% of the DNA alkylation products are monofunctional adducts on the N7-position of guanine and the N3-position of adenine, respectively, and approximately 17% are N7-position guanine bifunctional cross-links (Shakarjian et al., 2010). About 25% of the DNA cross-links are between complementary strands, whereas the rest are intra-strand (Walker, 1971). Evidence suggests that mustard-induced DNA cross-linking is not solely responsible for DNA breaks (Papirmeister et al., 1985). In fact, the monofunctional alkylation of purines also renders DNA sensitive to endonucleases, which induce DNA breaks. DNA breaks and adduct formation are associated with the activation of ataxia telangiectasia–mutated (ATM) and ataxia telangiectasia–related (ATR) protein kinases (Hurley and Bunz, 2007; Jowsey et al., 2009). These protein kinases phosphorylate a wide range of target proteins involved in transcriptional regulation, cell cycle progression, and DNA repair (Dillman et al., 2005; Rosenthal et al., 1998). Specifically, p53 responsive genes including cyclin G1 and MDM2 are upregulated after sulfur mustard exposure along with bax, EphA2, IEX1/IER3, genes regulating apoptosis, and the transcription factor, ATF3. Increases in metallothionein and Snk following mustard exposure may contribute to cell injury and mitosis (Burns et al., 2003; Dillman et al., 2005; Fan and Cherian, 2002).
9.2.3.1 Cytoto...