
eBook - ePub
Catalyst Handbook
Martyn V. Twigg
This is a test
Compartir libro
- 608 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
Catalyst Handbook
Martyn V. Twigg
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
This book bridges the gap between theory and practice. It provides fundamental information on heterogeneous catalysis and the practicalities of the catalysts and processes used in producing ammonia, hydrogen and methanol via hydrocarbon steam reforming. It also covers the oxidation reactions in making formaldehyde from methanol, nitric acid from ammonia and sulphuric acid from sulphur dioxide. Designed for use in the chemical industry and by those in teaching, research and the study of industrial catalysts and catalytic processes. Students will also find this book extremely useful for obtaining practical information not available in more conventional textbooks.
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es Catalyst Handbook un PDF/ePUB en línea?
Sí, puedes acceder a Catalyst Handbook de Martyn V. Twigg en formato PDF o ePUB, así como a otros libros populares de Physical Sciences y Chemistry. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
Chapter 1
Fundamental Principles
1.1. Fundamentals of Heterogeneous Catalysis
1.1.1. Introduction
The word “catalysis” is used to describe many phenomena but in all instances an agent (the catalyst) exerts a more-than-proportional influence over some change. Indeed, the word catalysis, coined by Berzelius in 1836 to describe some enhanced chemical reactions,1 is now used popularly in a non-technical way. In this book we shall restrict use of the term “catalysis” to the heterogeneous catalysis of gas-phase reactions; that is, the promotion of reactions between gases by the use of solid catalysts. We shall be concerned mainly with a few reactions of great importance in the heavy inorganic chemicals industry. The simplicity of the overall chemistry of these reactions usually hides a bewildering complexity of separate reaction steps on the catalyst surface. The discussion in this chapter is limited to a few general principles, which are illustrated by examples from the processes described in later chapters. Further study, essential for any full understanding of heterogeneous catalysis, can be undertaken using the books listed in Appendix 1. All of the topics of this chapter are treated comprehensively in a recent book by Richardson1a.
Catalysts are frequently defined as materials which accelerate chemical reactions without themselves undergoing change. As the manager of any plant using a catalytic process knows, this is too optimistic a definition: the properties of all real catalysts do change with use. The definition is also unsatisfactory in a more general way, for it implies that the acceleration is brought about without direct involvement of the catalyst in the process. Rather than attempting to produce an alternative succinct definition, we can list the essential features of the heterogenous catalysis of gas-phase reactions.
1. The presence of a solid material (the heterogeneous catalyst) changes the rate of an overall chemical reaction. The term “solid” is subject to some qualification, as the sulphuric acid catalyst (see Chapter 10) is a melt held in a porous solid and some other catalysts also contain mobile phases, e.g. some potassium salts in steam reforming catalysts (see Chapter 5). The overall chemical reaction concerns the gas-phase species only, but this does not preclude the involvement of the solid in the formation of intermediate species—indeed, this is essential for heterogeneous catalysis.
2. The products of the catalysed reaction can, at least in principle, be obtained from an uncatalysed reaction under the same conditions. There is, therefore, no way of using catalysis to “cheat” equilibrium. In practice, however, the uncatalysed reaction may be immeasurably slow or may give very different products.
3. Any useful catalyst must have a high productivity. Thus, we expect 1 tonne of catalyst to “make” many tonnes of product. Alternatively, to look at catalysis on the atomic scale, the catalysed reaction steps must occur many times at the reaction site on the catalyst surface before catalytic activity is lost.
4. The catalysed reaction steps take place very close to the solid surface. These steps may be between gas molecules adsorbed on the catalyst surface, or extensive reaction can take place involving the topmost atomic layers of the catalysts. The influence of the solid does not effectively extend more than an atomic diameter into the gas phase, and the direct involvement of atoms below the topmost layers is not usually possible.
1.1.2. The Role of Catalysis
The synthesis of ammonia and its subsequent oxidation to nitric oxide give illustrations of two ways in which catalysts are essential to the chemical industry.
1.1.2.1. Ammonia synthesis
Ammonia synthesis from nitrogen and hydrogen occurs by the overall reaction shown in equation (1).
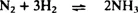 | (1) |
No reaction takes place with the reactants at ambient temperatures, and even with an active catalyst a temperature of some 400°C is needed to obtain commercially useful rates of reaction. Nothing happens in the system without a catalyst as the temperature is raised until, at temperatures higher than 1000°C, a significant proportion of the hydrogen molecules are dissociated into atoms, as shown in equation (2). For example, at 1430°C, with a pressure of molecular hydrogen of 150 bar, the partial pressure of atomic hydrogen would be 0.1% of the H2 pressure, i.e. 0.15 bar. Even this does not provide a mechanism for fixing nitrogen, for the reaction of hydrogen atoms with nitrogen molecules is very slow.2 Only above 3000°C, where the even more strongly-bound nitrogen molecules start dissociating to atoms, equation (3), does nitrogen fixation become possible.
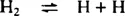 | (2) |
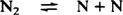 | (3) |
If each nitrogen atom formed in this way gave a molecule of ammonia by subsequent reactions with hydrogen molecules or atoms, then the rate of ammonia formation can be calculated from the rate of reaction (3).2 A 100 m3 reactor containing a 3:1 hydrogen/nitrogen mixture at a total pressure of 200 bar and a temperature of 3150°C would give 1300 tonnes of ammonia per day.
However, this simple analysis ignores the reverse reaction. Reaction (1) is an equilibrium reaction, and ammonia synthesis is favoured by low temperatures and high pressures. Thermodynamic data3 can be used to show that the partial pressure of ammonia under the conditions above cannot exceed 0.07 bar, so with a gas space velocity of 104 h−1, the make rate of ammonia would be only 6 tonnes day−1.
The role of the catalyst in ammonia synthesis is therefore that of making the reaction go sufficiently fast (by facilitating the dissociation of molecular nitrogen) so that significant rates are obtained under conditions where the equilibrium conversion is large enough to be useful (Figure 1.1).
1.1.2.2. Ammonia oxidation
The qu...