![]()
Yashin AI, Jazwinski SM (eds): Aging and Health - A Systems Biology Perspective.
Interdiscipl Top Gerontol. Basel, Karger, 2015, vol 40, pp 177-188 (DOI: 10.1159/000364982)
______________________
Conservative Growth Hormone/IGF-1 and mTOR Signaling Pathways as a Target for Aging and Cancer Prevention: Do We Really Have an Antiaging Drug?
Vladimir N. Anisimov
Department of Carcinogenesis and Oncogerontology, N.N. Petrov Research Institute of Oncology, St. Petersburg, Russia
______________________
Abstract
Inactivation of the GH/insulin/IGF-1 signaling molecules corresponding genes as well as the inactivation of serine/threonine protein kinase mTOR increases life span in nematodes, fruit flies and mice. Evidence has emerged that antidiabetic biguanides and rapamycin are promising candidates for pharmacological interventions leading to both life span extension and prevention of cancer. The available data on the relationship of two fundamental processes-aging and carcinogenesis-have been suggested to be a basis for understanding these two-side effects of biguanides and rapamycin.
© 2015 S. Karger AG, Basel
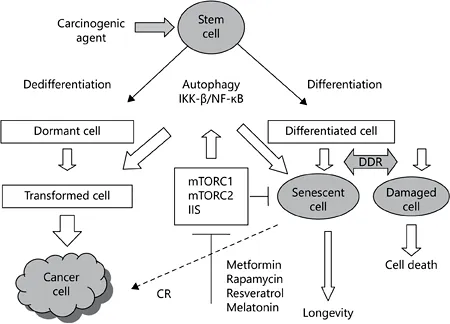
There are nine tentative hallmarks of aging in mammals, which may represent common denominators of aging in different organisms: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered cell-to-cell communication [1]. At the same time, there is also sufficient similarity in the patterns of changes observed during normal aging and the process of carcinogenesis (table 1) [2]. As can be seen in figure 1, DNA damage induced by environmental and endogenous carcinogenic factors [reactive oxygen species, ionizing radiation, ultraviolet, constant illumination (light at night), some diets, oncogenes, etc.] may lead to cellular senescence or cellular lesions which could be deleted by apoptosis. The same agents can induce damage which is followed by neoplastic transformation, thus leading to cancer [2, 3]. During the last decade, the intensive search for antiaging remedies has led to the conclusion that both insulin/IGF-1 signaling (IIS) and nutrient response pathways defined by the mTOR protein kinase pathways control aging and age-asso-ciated pathology in worms, insects and mammals [4]. In each of these organisms, genetic downregulation or interruption of this signaling pathway can lead to major extension of longevity. There are two functionally distinct mTOR complexes called mTORC1 and mTORC2. mTORC1 is activated by insulin and related growth factors through phosphatidylinositol-3-OH kinase and AKT kinase signaling and repressed by AMP-activated protein kinase, a key sensor of cellular energy status [4]. The mTORC1 is involved in promoting messenger RNA translation and protein synthesis through ribosomal protein S6 kinases (S6Ks) and 4E-BP protein, which in the hypophosphorylated form acts as a negative regulator of the cap-binding protein eIF4E. mTORC1 also stimulates lipid biosynthesis, inhibits autophagy, and through hypoxic response transcription factor HIF-1α regulates mitochondrial function and glucose metabolism. Rapamycin suppresses mTORC1 and indirectly mTORC2, which leads to metabolic lesions like glucose intolerance and abnormal lipid profile [4]. The phosphorylation of S6K1 at T389 by TORC1 is susceptible to rapamycin. The life span of S6K1 -deficient female mice increased by 19% in comparison to the wild-type controls without any effect on the incidence of tumor development [5]. It is worth noting that there was no significant effect of the protein knockout on the life span of male mice. These data suggest that S6K1 is involved in mammalian life span regulation downstream of TORC1. Taking into consideration the negative effect of rapamycin on glucose tolerance and liver insulin sensitivity [5], Lamming et al. [6] studied the effect of mTORC1 and mTORC2 regulator gene modification on the life span of mice. There was no increase in life span in either female or male mtor+/-, Raptor+/-, mlst8+/- or mtor+/- Raptor+/- mice. However, female mtor+/- mlst8+/- mice lived longer by 14.4% in comparison to wild-type mice. The longevity of male mtor+/- mlst8+/- mice was unaffected. Female mtor+/- mlst8+/- mice were not calorie restricted through reduced food intake or increased energy expenditure, and had normal body weights and levels of activity consistent with the phenotypic effects. mtor+/- mlst8+/- mice exhibited an approximately 30-60% reduction in the abundance of hepatic mTOR, Raptor, mLST8, and Rictor, whereas the expression of mTOR complex subunits was less affected in Raptor+/- and mtor+/- Raptor+/- heterozygotes. The authors believe that suppression mTORC1 signaling is sufficient for life span prolongation independent of changes in glucose homeostasis.
Table 1. Changes developing in organism during natural aging and carcinogenesis: effects of geroprotectors
Fig. 1. Relationship between aging and carcinogenesis: the key role of IIS and mTOR signaling. DNA damage induced by environmental and endogenous factors (reactive oxygen species, ionizing radiation, ultraviolet, constant illumination, some diets, oncogenes, etc.) may lead to cellular senescence or cellular lesions which could be deleted by apoptosis. The same agents can induce damage followed by neoplastic transformation, thus leading to cancer. Metformin, rapamycin, and some other compounds with mTOR and US-inhibitory potential (resveratrol, melatonin) are able to modify both aging and carcinogenesis. DDR = DNA damage response.
Calorie restriction (CR) is the only known intervention in mammals that has been consistently shown to increase life span, reduce incidence and retard the onset of age-related diseases, including cancer and diabetes. CR has also been shown to increase resistance to stress and toxicity, and maintain youthful levels of function and vitality in laboratory mammals at advanced chronological age [7]. CR in rhesus monkeys have produced physiological responses strikingly similar to those observed in rodents and delayed the onset of age-related diseases, but effects on longevity were not consistent [8]. Data from these studies indicate that long-term CR reduces morbidity and mortality in primates, and thus may exert beneficial ‘antiaging’ effects in humans. Although understanding the role of GH and IIS in the control of human aging is incomplete and somewhat controversial, available data indicate that dietary prevention of excessive IGF-1 and insulin secretion and using diet and exercise to enhance insulin sensitivity may represent the most hopeful approaches to cancer prevention and to extending human health span and life span [2-4]. Metformin, rapamycin, and some other compounds with mTOR and IIS-inhibitory potential (resveratrol, melatonin) are able to modify both aging and carcinogenesis. This chapter focuses on the effects of biguanides and rapamycin, whereas data on resveratrol and melatonin will be discussed elsewhere.
Antidiabetic Biguanides as Geroprotectors
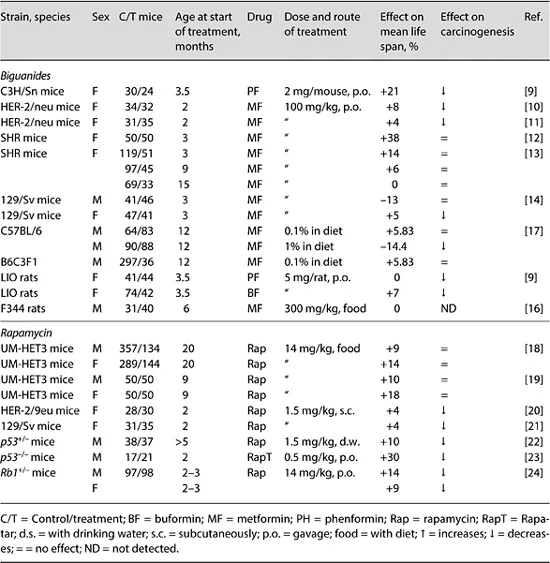
The concept of CR mimetics is now being intensively explored [3]. The antidiabetic biguanides, phenformin, buformin and metformin were observed to reduce hyperglycemia, improve glucose utilization, reduce free fatty acid utilization, gluconeogenesis, serum lipids, insulin, and IGF-1, reduce body weight and decrease metabolic immunodepression both in humans and rodents [3]. The results of studies on the effect of antidiabetic biguanides on the life span in mice and rats are summarized in table 2.
Table 2. Summary on effects of biguanides and rapamycin on life span and spontaneous carcinogenesis in rodents
The treatment with phenformin prolonged the mean life span of female C3H/Sn mice by 21% (p < 0.05) and the maximum life span by 26% in comparison with the controls [9]. At the time of death of the last mice in the control group, 42% of phenformin-treated mice were alive. The treatment with phenformin failed to influence the mean life span of female LIO rats; however, it increased the maximum life span by 3 months (10%) in comparison with the controls [9]. The treatment with phenformin slightly decreased the body weight of rats and delayed age-related switching off of estrous function. Similar findings were observed in female rats exposed to another bi-guanide, buformin [9]. Administration of metformin to female transgenic HER-2/ neu mice did not change the body weight or temperature; it slowed down the agerelated rise in blood glucose and triglyceride levels, decreased the serum level of cholesterol and β-lipoproteins, delayed the age-related irregularity in estrous cycle, extended the mean life span by 4-8% and the maximum life span by 1 month in comparison with the control animals [10, 11]. It is well known that excess of body weight and obesity leads to development of metabolic syndrome, type 2 diabetes, premature switching off of reproductive function and risk of cancer [2, 9]. The mechanism behind the geroprotective effect of metformin could reside in its ability to lower body weight.
Metformin increased the mean life span of the last 10% of survivors by 20.8% and the maximum life span by 2.8 months (10.3%) in female SHR mice in comparison with control mice [12]. The decreased body temperature and postponed age-related switching off of estrous function were observed in the group of metformin-treated mice. In another set of experiments, female SHR mice were given metformin from the age of 3, 9 or 15 months [13]. Metformin started at the age of 3 months increased the mean life span by 14% and maximum life span by 1 month, whereas the treatment started at the age of 9 months by 6%; metformin started at the age of 15 months did not affect life span.
The treatment with metformin slightly modified the food consumption but failed to influence the dynamics of body weight. Metformin decreased by 13.4% the mean life span of male 129/Sv mice and slightly increased the mean life span of females (by 4.4%). Metformin failed to influence spontaneous tumor incidence in male 129/Sv mice, decreased 3.5-fold the incidence of malignant neoplasms in female mice, while somewhat stimulated formation of benign vascular tumors [14].
Significant prolongation (by 20.1%) of the survival time was observed in male (but not female) transgenic mice with Huntington's disease without affecting fasting blood glucose levels. Increasing the dose of the drug did not improve the survival of mice [15]. In the NIA study [16], 6-month-old male F344 rats were randomized to one of four diets: control, CR, diet supplemented with metformin and standard diet pair fed to metformin. There were no significant differences in the mean life...