![]()
1 Transposons for Insect Transformation
David A. O’Brochta,1,2,3* Kasim George2,3 and Hanfu Xu4
1.1 Transposable Elements
Transposable elements were first discovered because of their role in hypermutable phenotypes and their effects on chromosomal integrity (McClintock, 1951). While initially thought to be enigmatic and minor constituents of genomes, modern genomics has revealed the extent to which genomes are composed of DNA sequences whose origins can be traced directly to transposable elements (Feschotte and Pritham, 2007). In many instances the majority of DNA in genomes is of transposable element origin, and their abundance within genomes is due in part to the strong tendency of transposable elements to increase in copy number as a consequence of their excision and transposition activity (Feschotte and Pritham, 2007). One large class of transposable elements moves by transcribing an RNA copy of the element, converting it to DNA and then integrating the DNA copy into a new genomic location. These copy-and-paste transposable elements, or retrotransposons, include those with and without long repeat sequences at their termini and all can rapidly increase in copy number when they are active within a genome (Gogvadze and Buzdin, 2009). Another class of transposable elements, the cut-and-paste transposable elements, or DNA transposons, do not move using an RNA intermediate but, instead, the elements are precisely excised from the genome and then reinserted at new genomic locations. Although this mechanism is inherently conservative and does not in itself result in an increase in element copy-number, cellular mechanisms to repair the resulting double-stranded gap in chromosomal DNA can result in copies of the element being restored to its original position resulting in a net gain in copy number following element movement (Munoz-Lopez and Garcia-Perez, 2010). Transposition of cut-and-paste transposable elements during S phase can also result in an increase in element copy number if elements excise and then transpose ahead of replication forks (Chen et al., 1992). Consequently, actively transposing DNA transposons, like retrotransposons, can contribute to expansions in the size of genomes. While the dynamics of transposable element movement and the resulting impacts on genome evolution have been extensively investigated and discussed, it is their natural abilities to integrate into chromosomes and move within genomes that has made them vitally important genetic platforms upon which to construct powerful genetic technologies for genome analysis (Ivics and Izsvak, 2010; Munoz-Lopez and Garcia-Perez, 2010). Today, transposable elements are the foundation upon which many other genome manipulation technologies are based, and as the accumulation of insect genomic DNA sequence data continues to increase there will be a growing demand to adapt existing transposon-based technologies and to develop new ones that are applicable to a wide variety of insect species (Ivics and Izsvak, 2010). Here we will review briefly the characteristics and properties of the transposable elements that have been shown to be active in insects and upon which various genetic technologies have been assembled, followed by a brief description of those technologies and where they have been applied in insect biology.
1.2 DNA Transposons
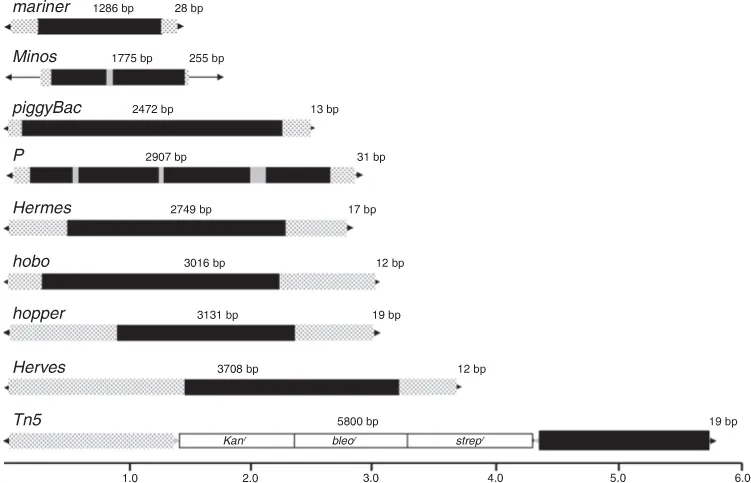
DNA transposons are a diverse class of transposable elements that have fairly simple organizations and modes of transposition (Craig et al., 2002). In general, the elements are usually less than 10 kb in length and often less than 5 kb. The parent element of a given type of DNA transposon usually has a single transcription unit that encodes for a protein, the transposase, which is responsible for mediating excision and transposition (cutting-and-pasting). While there is great variation in the amino acid sequences of known transposase proteins, transposases are currently thought to have evolved from a common ancestral protein with integrase activity (Eickbush and Malik, 2002). Evidence supporting this hypothesis consists of the almost invariant presence of three amino acids (two aspartic acids and a glutamic acid, DDE) in the catalytic site of most transposases, as well as a conserved structure of the catalytic domain of these proteins referred to as the integrase fold (Nesmelova and Hackett, 2010; Montano and Rice, 2011). In addition to protein coding sequences, elements are composed of non-coding sequences, some of which play an important structural role in the excision and transposition reactions. DNA transposons are delimited by terminal sequences that are repeated at each end of the element in opposite orientations – inverted terminal repeats. Inverted terminal repeat sequences can range from fewer than 20 bp to over 200 bp, depending on the specific element and they serve as DNA binding and signal sequences, directing transposase and ensuring DNA cleavage reactions occur at the correct place (Hickman et al., 2010) (Fig. 1.1).
DNA transposons are found in three basic forms: complete (autonomous) elements as just described, including all structural and transposase-coding sequences; incomplete (non-autonomous) elements that lack a complete transposase coding region but have intact structural elements allowing them to transpose if functional transposase is provided in trans; and defective elements that lack structural integrity, preventing them from further transposition. Elements integrate into short regions of DNA by creating staggered double-stranded breaks resulting in single-strand overhangs with the length of the staggered DNA breaks and single-strand overhangs varying from 2 to 9 bp in length, as is characteristic of individual elements (Curcio and Derbyshire, 2003). Upon ligation of the element into the double-stranded gap in the target molecule the single-stranded overhangs are repaired resulting in target site sequences flanking the integrated transposon. Consequently, transposon integration invariably results in the creation of a short duplication of the target sequence (Curcio and Derbyshire, 2003). The first step in the transposition reaction, transposon excision, involves the precise removal of the element from the donor DNA molecule. However, the double-stranded break in the donor chromosome must be repaired using the cell’s endogenous gap-repair machinery involving homologous recombination or non-homologous-end-joining. Non-homologous-end-joining almost always results in the imperfect restoration of the original integration site to its pre-integration condition. The ‘footprint’ left by the excised element may be a copy of the duplicated target site, the insertion of extra nucleotides at the ends of the broken chromosome or the asymmetrical deletion of DNA prior to end joining. Alternatively, homologous recombination-based repair can result in a copy of the transposon being restored to its original location resulting in a net gain of one copy in the genome. These post-excision repair processes are observed following the movement of all DNA transposons to a greater or lesser extent depending on the element. While the molecular details of the excision and integration reactions are beyond the scope of this chapter, it is worth noting that there are subtle but significant variations from element to element, but overall, cut-and-paste elements move using very similar mechanisms (Craig, 1995; Curcio and Derbyshire, 2003).
Fig. 1.1. Transposons active in insect germ-lines and used as platforms for genome manipulation. Fully intact (autonomous) elements are drawn with the length of the element indicated and with a scale in kilobases (0–6 kb) shown. The sequences comprising most elements belong to one of three general categories: inverted terminal repeats (arrows), non-coding sequences (stippled) and transposase-coding sequences (black, coding sequences and grey, introns).
The simple structure and biochemistry associated with DNA transposons has made them particularly amenable to being used as functional genomics tools. Attaching the non-coding structural sequences of a transposon to any DNA sequences confers on that sequence many of the mobility characteristics of the parent transposon when the appropriate transposase is present. This observation is the basis upon which DNA transposons have been converted into mobile DNA platforms capable of being modified in a variety of ways and of carrying functional DNA sequences for a variety of applications (Ivics and Izsvak, 2010).
1.3 Transposons with Activity in Insects
1.3.1 P
P elements were the first transposable elements to be isolated from insects and were originally discovered in Drosophila melanogaster as the paternal genetic factors found in some wild-caught lines that induced a genetic syndrome known as hybrid dysgenesis when introduced into females from different wild-caught lines (Kidwell et al., 1977) (Fig. 1.1). P elements have a somewhat typical organization of DNA transposons and are 2907 bp in length with 31 bp terminal inverted repeats and 8 bp integration target sites. DNA sequences within the element and located adjacent to the terminal inverted repeat are also important for element movement (Rio, 2002; Castro and Carareto, 2004). The transposase of P elements is encoded in four exons and expression is limited to the germ-line as a consequence of a sex-specific splicing event. P transposase appears quite different from the transposases found in other DNA transposon families and it is not obvious that it is a DDE-type transposase (Hickman et al., 2010). The evolutionary history of P elements in insects has been the subject of much study and it is clear that P elements isolated from D. melanogaster were only recently acquired by this species (Clark et al., 1994; Loreto et al., 2012). Phylogenetic and DNA sequence evidence convincingly show that P elements currently in D. melanogaster were acquired by an unknown mechanism from D. willistoni (Clark et al., 1994). Following its horizontal transfer into the genome of D. melanogaster, it quickly spread within and among populations, and within the last century has spread throughout all of the D. melanogaster populations of the world (Engels, 1997). The high levels of transpositional activity of P elements have made them particularly useful as platforms for constructing genetic technologies in D. melanogaster (Ryder and Russell, 2003). With one exception, its activity has not been reported in insect species outside the family Drosophilidae (O’Brochta and Handler, 1988; Kim et al., 2003). P elements have shown little evidence of mobility in non-drosophilid insect species and this extremely limited host range is uncommon among cut-and-paste DNA transposons isolated from insects (Table 1.1). The specific basis for the P element’s extreme intolerance of non-drosophilid hosts is unknown.
The behaviour of P elements and their patterns of movement within the genome of D. melanogaster are fairly typical for DNA transposons. The sequence of the 8 bp target site used by P elements is highly variable and shows little evidence for strict nucleotide requirements (Liao et al., 2000). At the nucleotide level, the element shows very low target-site specificity. But the patterns of P element integration in D. melanogaster are distinctly non-random when considered at the level of individual chromosomes or genes. Integration patterns within the genome show clear signs of preferred and non-preferred regions of insertion (Bellen et al., 2011). Chromatin structure appears to play some role in determining this distribution pattern and elements tend to accumulate in the 5′ region of expressed genes and more recently have been found also to prefer origins of replication (Spradling et al., 2011). Both regions are expected to have open chromatin configurations. Integrated P elements can be readily induced to remobilize in the presence of functional P transposase, but elements tend to relocate to linked sites and sites close to the original integration site in a phenomenon known as ‘local hopping’. P element transposition activity also depends on the size of the element, with elements carrying large amounts of DNA transposing at lower rates than smaller elements. P elements carrying more than 10 kb tend to be difficult to work with because their rates of transposition tend to be impractically low (Spradling, 1986). The DNA sequences and genes contained within P elements can also influence the elements’ patterns of integration. Some DNA sequences have been shown to strongly bias the site of integration to regions with sequence similarity to internal sequences, a phenomenon sometimes referred to as ‘transposon homing’ (Taillebourg and Dura, 1999; Bender and Hudson, 2000). P element excision results in double-stranded breaks in the donor chromosome, which are then subsequently repaired. Gap repair following P element excision is occasionally by non-homologous-end-joining, resulting in the frequent addition or deletion of sequences flanking the insertion site (Castro and Carareto, 2004). This property of P element movement has been used to great advantage by those wishing to create mutations in D. melanogaster. P elements inserted near genes can be induced to excise, and in some cases, excision-associated deletions will disable neighbouring genes and create null mutations. Alternatively, P element excision-ca...