New Therapeutics for Traumatic Brain Injury
Prevention of Secondary Brain Damage and Enhancement of Repair and Regeneration
Kim Heidenreich
- 352 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
New Therapeutics for Traumatic Brain Injury
Prevention of Secondary Brain Damage and Enhancement of Repair and Regeneration
Kim Heidenreich
À propos de ce livre
New Therapeutics for Traumatic Brain Injury: Prevention of Secondary Brain Damage and Enhancement of Repair and Regeneration explores traumatic brain injury (TBI), a major cause of death and disability throughout the world. The delayed nature of the secondary injury phase suggests that there is a therapeutic window for pharmacological interventions or other approaches to prevent progressive tissue damage and improve functional outcomes. It is now apparent that therapeutic interventions should entail both protective and repair/regeneration strategies depending on the phase of brain injury.
This book describes emerging experimental strategies for the treatment of TBI, including new anti-inflammatory or anti-apoptotic therapeutics that limit brain damage, and novel or repurposed drugs that enhance repair or regeneration of the brain after injury.
- Comprehensive overview of basic approaches and translational development of new therapies for TBI
- Edited by a prominent TBI researcher that includes contributions by leading global researchers in the field
- Presents a great resource for researchers and practitioners to learn more about the many evolving preclinical studies and clinical trials currently underway, and the challenges of bringing translational studies in TBI to the clinic
Foire aux questions
Informations
Why Did the Phase III Clinical Trials for Progesterone in TBI Fail? An Analysis of Three Potentially Critical Factors
Abstract
Keywords
Introduction: Progesterone Treatment Showed Promise in Preclinical Research
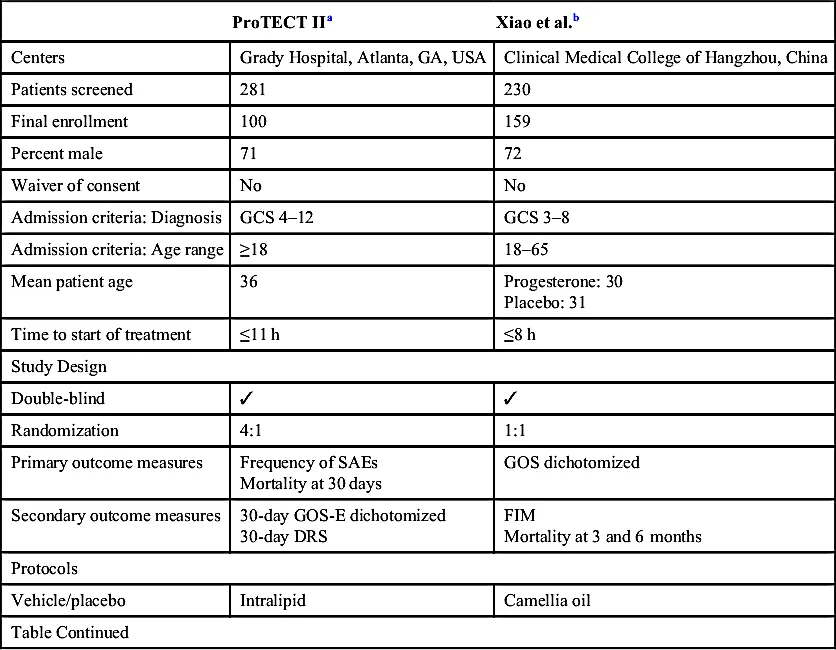
The Phase II Trials
| ProTECT IIa | Xiao et al.b | |
| Centers | Grady Hospital, Atlanta, GA, USA | Clinical Medical College of Hangzhou, China |
| Patients screened | 281 | 230 |
| Final enrollment | 100 | 159 |
| Percent male | 71 | 72 |
| Waiver of consent | No | No |
| Admission criteria: Diagnosis | GCS 4–12 | GCS 3–8 |
| Admission criteria: Age range | ≥18 | 18–65 |
| Mean patient age | 36 | Progesterone: 30 Placebo: 31 |
| Time to start of treatment | ≤11 h | ≤8 h |
| Study Design | ||
| Double-blind | ✓ | ✓ |
| Randomization | 4:1 | 1:1 |
| Primary outcome measures | Frequency of SAEs Mortality at 30 days | GOS dichotomized |
| Secondary outcome measures | 30-day GOS-E dichotomized 30-day DRS | FIM Mortality at 3 and 6 months |
| Protocols | ||
| Vehicle/placebo | Intralipid | Camellia oil |
| Table Continued | ||
| ProTECT IIa | Xiao et al.b | |
| Route of administration | i.v. | i.m. |
| Test drug administration | Total: 3 days at 12 mg/kg/day: Loading dose 0.71 mg/kg/h at 14 mL/h for 1 h, then 10 mL/h of 0.5 mg/kg/h for 11 h, then 5 doses at 10 mL/h to deliver 0.5 mg/kg/h for 11 h | Total: 5 days 1 mg/kg, then once every 12 h for 5 days at 2 mg/kg/day A single-dose volume of 0.05 mL/kg over 5 consecutive days |
| Findings | ||
| Mortality/morbidity | 30 days Severe progesterone 13.2% Severe placebo 40.0% Moderate progesterone 16.7% Moderate placebo 14.3% | 6 months Progesterone 18% Placebo 32% |
| Functional recovery | GOS at 30 days Severe progesterone 21.2% Severe placebo 26.7% Moderate progesterone 55.6% Moderate placebo 0% DRS mean total at 30 days Severe progesterone 10.7 Severe placebo 4.4 Moderate progesterone 5.0 Moderate placebo 12.7 | GOS score at 3 months Progesterone 47% Placebo 31% GOS ... |
Table des matières
- Cover image
- Title page
- Table of Contents
- Copyright
- List of Contributors
- Foreword
- Preface
- Introduction
- Part I. Interventional Therapies for TBI Previously or Currently in Phase 3 Clinical Trials
- Part II. Repurposing FDA Approved Drugs for TBI Treatment
- Part III. Interventional Drugs for TBI in Phase 1–2 Clinical Trials
- Part IV. Interventional Drugs for TBI in Preclinical Development
- Part V. Drugs for TBI Rehabilitation
- Index