Biological Sciences
Entamoeba Histolytica
Entamoeba histolytica is a parasitic amoeba known to cause amoebiasis, a potentially serious intestinal infection in humans. It is transmitted through ingestion of contaminated food or water. The amoeba can invade the intestinal wall, leading to symptoms such as diarrhea, abdominal pain, and in severe cases, liver abscesses. Proper sanitation and hygiene are crucial for prevention.
Written by Perlego with AI-assistance
Related key terms
1 of 5
12 Key excerpts on "Entamoeba Histolytica"
- eBook - ePub
- Fabrizio Bruschi(Author)
- 2017(Publication Date)
- Bentham Science Publishers(Publisher)
Entamoeba Histolytica and other Pathogenic Intestinal AmoebeaIntroduction
Entamoeba Histolytica is an extracellular anaerobic parasitic enteric protozoan and the etiologic agent of amebic colitis and liver abscesses through water- and food-borne transmissions. Infection with E. histolytica, named amebiasis, affects up to 10% of the world’s population (approximately 500 million people worldwide) and is a major cause of symptomatic illnesses (50 million of persons/year) and death (100,000 persons/year). Although the incidence of amebiasis may be over-estimated by misdiagnosing pathogenic E. histolytica with non-pathogenic amoebas (e.g., E. dispar and E. moshkovskii), amebiasis is considered one of the major causes of death due to parasitic infections in developing countries, after malaria and schistosomiasis [1 ].History
Reports of people with bloody and mucous diarrhoea (probably amebiasis) were written 1000-600BC when Assyrian and Babylonian texts mentioned cases of dysentery in the Tigris-Euphrates basin [2 ]. By the 16th century, amebiasis was commonly described in developed Europe, mostly due to the growth of European colonies and increased world trade. At that time, there was a distinction of hepatic and intestinal forms of amebiasis. The first accurate description of both forms of the disease came from the book “Researches into the Causes, Nature and Treatment of the More Prevalent Diseases of India and of Warm Climates Generally” written by James Annersley in the 19th century [2 ]. With advances in microscopy, Friedrich Losch was the first to describe E. histolytica in Russia in 1873 from a young farmer who had been suffering from chronic dysentery [2 ]. In 1925, Emile Brumpt suggested that there must be two species: one that is invasive while the other is not (later named E. dispar) to explain why some people who were infected with E. histolytica (i.e. cysts in their stool) could never develop symptoms of the disease and spontaneously clear the infection [3 ]. However, it was not until the 1990’s, due to biochemical, immunological and genetic tools that E. histolytica was reclassified as an invasive species named E. histolytica and the non-invasive species named E. dispar [3 - eBook - ePub
- Mark F Wiser(Author)
- 2010(Publication Date)
- Garland Science(Publisher)
E. dispar (or both) and in many tropical countries the prevalence may approach 50%. There are an estimated 50 million clinical cases of amebiasis per year with up to 100 000 deaths.Life Cycle and Morphology
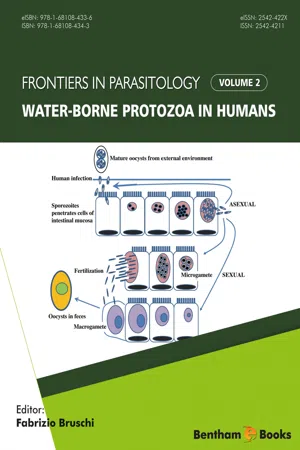
E. histolytica exhibits a typical fecal–oral life cycle consisting of infectious cysts passed in the feces and trophozoites which replicate within the large intestine. The infection is acquired through the ingestion of cysts and the risk factors are similar to other diseases transmitted by the fecal–oral route (see Chapter 2 ). Contaminated food and water are probably the primary sources of infection. The higher prevalence in areas of lower socioeconomic status is likely due to poor sanitation and a lack of indoor plumbing. However, E. histolytica is rarely the cause of travelers’ diarrhea and is usually associated with long-term (>1 month) stays in an endemic area. A higher prevalence of E. histolytica infection is also observed in institutions, such as mental hospitals, orphanages, and prisons, where crowding and problems with fecal contamination are contributing factors. A high prevalence among male homosexuals has also been noted in several studies.Upon ingestion the cysts pass through the stomach and excyst in the lower portion of the small intestine. Excystation involves a disruption of the cyst wall and the quadrinucleated ameba emerges through the opening. The ameba undergoes a round of nuclear division followed by three successive rounds of cytokinesis (i.e., cell division) to produce eight small uninucleated trophozoites, sometimes called amebula. These trophozoites colonize the large intestine, especially the cecal and sigmoidorectal regions, where they feed on bacteria and cellular debris and undergo repeated rounds of binary fission. Like many other intestinal protozoa, Entamoeba trophozoites are obligate fermenters and lack enzymes of the tricarboxylic acid cycle and proteins of the electron transport chain. In keeping with this anaerobic metabolism the parasites also lack mitochondria and only have a mitochondrial remnant called a mitosome. Interestingly, E. histolytica - eBook - ePub
- Marianne D. Miliotis, Jeffrey W. Bier(Authors)
- 2003(Publication Date)
- CRC Press(Publisher)
26
Entamoeba Histolytica
Terry F. H. G. Jackson and Selvan G. ReddySouth African Medical Research Council, Durban, South AfricaI. BACKGROUND
Entamoeba Histolytica is a protozoan parasite responsible for the disease amebiasis. Although the organism generally asymptomatically parasitizes the human gastrointestinal tract, it can invade the intestinal mucosa, giving rise to amebic dysentery, and in some cases it spreads to other organs, primarily the liver, where it causes amebic liver abscess. It is estimated that amebiasis is responsible globally for approximately 70,000 deaths per annum and is the fourth leading cause of death due to a protozoan infection after malaria, Chagas’ disease, and leishmaniasis (1 ).The first definite description of Entamoeba Histolytica can be attributed to Fedor Losch in 1875 (2 ). Lösch described the clinical and autopsy findings of a case of fatal dysentery in a Russian migrant laborer. He recorded detailed descriptions of the amebae found in the stool and intestinal ulcers. He described their size, varying shape, their biphasic cytoplasm, the presence of ingested foreign bodies, among which he recognized red blood corpuscles, the characteristics of the nucleus, and the peculiar movements of the organisms. He concluded that these amebae were different from those known at that time and decided that they were a new species for which he proposed the name Amoeba coli. Although he was able to successfully infect dogs using stools from the autopsy and observed ulcerative lesions similar to that found in the migrant, Lösch did not attribute any etiological significance to the presence of these amebae in stools and lesions. Over the next 30 years, several authors added to these observations:In 1891 Councilman and Lafleur (3 ) reported on detailed studies of lesions found in intestines and hepatic abscesses. They confirmed the pathogenic role of Amoeba coli - eBook - PDF
Microbiology of Waterborne Diseases
Microbiological Aspects and Risks
- (Author)
- 2004(Publication Date)
- Academic Press(Publisher)
Entamoeba gingivalis inhabits the mouth and has been associated with periodontal disease (Lyons et al ., 1983), while E. polecki is of uncertain pathogenicity (Levine and Armstrong, 1970). However, E. histolytica is the predominant pathogenic amoeba and causes amoebic dysentery (amoebiasis) (Brumpt, 1925; Sargeaunt et al ., 1978). E. histolytica is indistinguishable by microscopy from the non-pathogenic E. dispar and the differentiation is rarely made in routine clinical microbiological diagnoses. Historically, since these organisms could not be differentiated, both were referred to as E. histolytica , and it has been estimated that about 10% of the world’s population are infected (Walsh, 1988) . However, 90% of these infections are asymptomatic and probably with the non-pathogenic E. dispar . Despite this there remains a considerable burden of disease and more than 100 000 deaths occur annually from invasive amoebiasis, making it the third leading parasitic cause of death in developing countries (Reed, 1992). The life cycle of E. histolytica has been described by Dobell (1928) and comprises an infective cyst form, metacyst, metacystic trophozoite, motile feeding trophozoite and precyst stages. The cyst form (10–16 m), which develops only in the intestinal tract, is shed in the faeces and is capable of survival in food and water. The mature cysts, which contain four nuclei, are transmitted to humans by the ingestion of faecally-contaminated food, water or from body contact. The amoebae within the mature cyst are activated by the neutral or alkaline environment in the small intestine, and separate from the cyst wall which is digested by enzymes within the gut lumen. Rapid nuclear and cytoplasmic division results in eight uninucleate trophozoites. The trophozoites (20–40 m) migrate to the large intestine where they multi-ply by binary fission and feed on the bacteria of the intestinal flora and on cell debris. - Gauri Misra, Vijay Kumar Srivastava(Authors)
- 2020(Publication Date)
- Academic Press(Publisher)
Chapter 1Amebiasis
Nurulhasanah Othman, Jorim Anak Ujang, Yee Ling Ng, Gaayathri Kumarasamy, and Rahmah Noordin Institute for Research in Molecular Medicine (INFORMM), Universiti Sains Malaysia, Gelugor, Penang, MalaysiaAbstract
Amebiasis is caused by Entamoeba Histolytica that remains a global health burden mainly in developing countries despite advancements in neglected tropical disease control programs by WHO. Patients with symptomatic amebiasis commonly present with amebic colitis and amebic liver abscess though most infected individuals are asymptomatic. The latter is the reservoir of this protozoan parasite and eventually causes the disease transmission. Therefore the new strategy in developing drugs and vaccines that can block disease transmission is important because only a few drugs are available in the market and the absence of effective vaccines to combat amebiasis. To accomplish that, a new diagnostic tool that can differentiate E . histolytica infection stages and nonpathogenic Entamoeba would be highly recommended.Keywords
Amoebiasis; Drug; Molecular pathway; Vaccine1.1. Introduction
1.1.1. History
Amebiasis was first reported as a deadly disease in 1873 by Hippocrates who examined a patient suffering from bloody dysentery [1 ]. Two years later, Entamoeba Histolytica trophozoite was identified by Fedor Aleksondrovich Losch in a farmer who suffered from a fatal case of dysentery [2 ]. Further investigation by inoculating the stool of the patient into the rectum of a dog caused a similar manifestation [2 ]. A significant milestone was achieved with the characterization of E . histolytica as the causative agent for amebic colitis and amebic liver abscess (ALA) in the 1890s by Sir William Olser and his colleagues [1 ]. Subsequently, the identification of cyst as an infectious stage was confirmed by Walker and Sellards in 1913 and followed by the establishment of the E . histolytica life cycle by Dobell in 1925 [1 ]. In 1997, amebiasis was ranked second as death-causing parasitic infection, after malaria [3 ]. Approximately 40,000–100,000 deaths occurred annually, which include 1.9%–9% of amebic colitis patients [4- eBook - ePub
- Lynne Shore Garcia(Author)
- 2016(Publication Date)
- ASM Press(Publisher)
3 ). Only malaria and schistosomiasis surpass amebiasis as leading parasitic causes of death.At least 11 amebae are found in the mouth or intestinal lumen (E. histolytica , Entamoeba dispar , Entamoeba moshkovskii , Entamoeba bangladeshi , E. hartmanni , E. coli , Entamoeba polecki , Entamoeba gingivalis , Endolimax nana , Iodamoeba bütschlii , and Blastocystis spp.). Of these, only E. histolytica and Blastocystis spp. have been considered to be pathogenic; however, E. moshkovskii and E. bangladeshi have been implicated as causing disease in humans, particularly in infants (4 ).E. histolytica versus E. disparAlthough a large number of people throughout the world are infected, only a small percentage will develop clinical symptoms. Morbidity and mortality due to E. histolytica vary, depending on the geographic area, the organism strain, and the patient’s immune status. For many years, the issue of pathogenicity has been very controversial, with essentially two points of view. Some thought that what was called E. histolytica was really two separate species of Entamoeba , one being pathogenic and causing invasive disease and the other being nonpathogenic and causing mild or asymptomatic infections. Others thought that all organisms designated E. histolytica were potentially pathogenic, with symptoms depending on the result of host or environmental factors, including the intestinal flora.In 1961, with the development of successful axenic culture methods requiring no bacterial coculture, sufficient organisms could be obtained for additional studies. Approximately 15 years later, reports indicated that E. histolytica clinical isolates could be classified into groups by using starch gel electrophoresis and review of banding patterns related to specific isoenzymes (5 ). The four isoenzymes are glucophosphate isomerase, phosphoglucomutase, malate dehydrogenase, and hexokinase. Sargeaunt concluded from this work that there are pathogenic and nonpathogenic strains (zymodemes) of E. histolytica - eBook - PDF
- Dwight D. Bowman, Charles M. Hendrix, David S. Lindsay, Stephen C. Barr(Authors)
- 2008(Publication Date)
- Wiley-Blackwell(Publisher)
Humans are host to several species of ameba, but only one species, Entamoeba Histolytica, is a seri-ous pathogen of humans, in which it causes large-bowel disease and occasionally hepatic or other deep tissue abscesses. Cats do not appear to be typ-ical hosts of this pathogenic ameba, even though Fig. 1.64. These organisms are found throughout the world but are more common in the tropics. There is only a single report in which amebae specifically iden-tified as Entamoeba Histolytica have been found in naturally infected cats. Location in Host Entamoeba Histolytica is a parasite of the mucosa of the large intestine. Some strains are more path-ogenic than others and are capable of causing ulcers within the mucosa and being carried by the bloodstream to other organs, for example, the liver, lung, and brain, where abscesses develop. In experimentally infected cats, abscesses have been observed to develop in the liver. Parasite Identification The 8 to 30 µ m trophozoite in a fecal smear pre-pared from fresh feces in physiologic saline will continue to be motile, and finger-shaped, rapidly extended pseudopodia can be observed. The trophozoites of Entamoeba Histolytica are often seen to contain ingested red blood cells. If the fecal material is stained with a trichrome stain or iron hematoxylin, the characteristic morphology of the nucleus with its small central karyosome and peripheral chromatin granules can be observed. The spherical cyst, 10 to 20 µ m in diameter, is more likely to be observed in formed feces. A fully developed cyst will contain four nuclei, although maturing cysts can contain anywhere from one to four nuclei. The cyst may contain elongate rod-shaped structures, chromatoidal bodies, which will have blunt ends. Life Cycle In humans, the typical life cycle includes the feeding, trophozoite stage that is found on or in the mucosa of the large bowel. - eBook - ePub
- Peter D. Walzer, Robert M. Genta(Authors)
- 2020(Publication Date)
- CRC Press(Publisher)
E. histolytica cysts (which are resistant to chlorination). Current research efforts directed at elucidating the molecular basis of disease pathogenesis, encystment, host intestinal factors, and host immunity may provide a means for future pharmacological or immunological prophylaxis. Pending the development of an effective vaccine or the availability of financial resources to improve public sanitation, amebiasis will remain a serious endemic disease in the population of the developing world as well as within high risk groups in developed countries.Acknowledgments
William A. Petri, Jr. is a Lucille P. Markey Scholar; Jonathan I. Ravdin was a Hartford Foundation Fellow. We thank Mutsuko Kennedy and Cynthia Kogut for excellent secretarial assistance.References- 1. Bloomfield, A. L. A bibliography of internal medicine: amebic dysentery. J. Chronic Dis. 5:235-252 (1957).
- 2. Stilwell, G. G. Amebiasis: its early history. Gastroenterology 28:606-622 (1955).
- 3. Kean, B. H., Mott, K. E., and Russel, A. J. Tropical Medicine and Parasitology: Classic Investigations. Cornell University Press, Ithaca, NY, 1978, pp. 71-168.
- 4. Osier, W. On the amoeba coli in dysentery and in dysenteric liver abscess. Johns Hopkins Hosp. Bull. 1:53-54 (1890).
- 5. Simon, F. Abscess of the liver, perforation into the lung; amoeba coli in sputum. Johns Hopkins Hosp. Bull. 1:97 (1890).
- 6. Councilman, W. T., and Lafleur, H. A. Amoebic dysentery. Johns Hopkins Hosp. Rep. 2:395-548 (1891).
- 7. Rogers, L. The rapid cure of amoebic dysentery and hepatitis by hypodermic injections of soluble salts of Emetime. Br. Med. J. 1:1424-1425 (1912),
- 8. Walker, E, L., and Sellards, A. W. Experimental entamoebic dysentery. Phillip. J. Sci. B. Trop. Med. 8:253-331 (1913).
- 9. Boeck, W. C., and Drbohlav, J. The cultivation of Entamoeba Histolytica. Am. J. Hyg. 5:371-407 (1925).
- 10. Dobell, C. Researches on the intestinal protozoa of monkey and man. Parasitology 20:357-412 (1928).
- 11. Diamond, L. S. Axenic cultivation of Entamoeba Histolytica
- eBook - PDF
- Julius P. Kreier, John R. Baker(Authors)
- 2012(Publication Date)
- Academic Press(Publisher)
They include both nonspecific and specific measures. Nonspecific measures include the improvement of water supplies and of excreta disposal, the adoption of more careful personal hygiene practices, and general social and economic development. It should be realized, however, that measures such as the upgrading of water supplies and sanitation are costly and are likely to be long-term undertakings. Specific measures that should be implemented wher-ever possible include community surveys and monitoring of levels of amebiasis, the rapid diagnosis and adequate treatment of patients with amebiasis, and surveil-lance and control of situations that may favor the spread of the disease. II. Amebas Other Than Entamoeba Histolytica The genus Entamoeba encompasses several species of human parasites: E. his-tolytica Schaudinn, 1903; E. hartmanni Von Prowazek, 1912; E. coli (Grassi, 1879) Hickson, 1909, and E. gingivalis (Gros, 1849) Smith and Barrett, 1914. Of these amebas, E. histolytica is the only important cause of disease. A relationship of E. gingivalis to periodontal disease still remains to be demonstrated (Linke et al., 1989). The characters currently of value for the differentiation of Entamoeba from other amebas have been summarized by Neal (1988). These include intrinsic characteristics such as morphology, type of nuclear division, type of movement, physiological characters, antigenic characteristics, D N A characteristics, isoen-zyme profiles and drug susceptibility, and extrinsic characteristics such as host specificity, factors affecting virulence, behavior in laboratory hosts, and clinical effects. The classification of the species of Entamoeba is based on the number of nuclei in their mature cysts, whether eight, four, or one (Neal, 1966). Entamoeba 3. Parasitic Amebas of the Intestinal Tract 123 coli belongs to the octonucleate cyst group. The quadrinucleated cyst group includes E. histolytica and E. - eBook - PDF
- David Evered, Geralyn M. Collins, David Evered, Geralyn M. Collins(Authors)
- 2009(Publication Date)
- Wiley(Publisher)
It is tempting to speculate that most trophozoites have the potential for virulence and that certain conditions may trigger its expression. The wider implications about the role of bacteria in the establishment of E. histofytica colonies and in the subsequent invasion of the colonic mucosa are far from being understood. On the one hand, certain bacteria that are not recognized or taken up by the amoebae may hinder the direct contact and attachment of the trophozoite to the apical surface of the intestinal epithelial cell (Martinez-Palomo 1982). On the other hand, bacterial strains that readily adhere to and are ingested by the amoebae may serve both as focal points of attachment to the intestinal mucosa and as triggers for enhanced trophozoite virulence. The nature of the intestinal microbial flora may thus have considerable importance for the pathogenicity of the amoebae and for the aetiology of the disease. Acknowledgement Our studies were supported by a grant from the Rockefeller Foundation. (Bicozamycin was obtained from Dr Kradoifer, Ciba-Geigy, Basel.) VIRULENCE OF Entamoeba Histolytica 17 REFERENCES Bar-Shavit 2, Ofek I, Goldman R, Mirelman D, Sharon N 1977 Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun 78:455-460 Bar-Shavit Z, Goldman R, Ofek I, Sharon N, Mirelman D 1980 Mannose-binding activity of Escherichia coli: a determinant of attachment and ingestion of the bacteria by macrophages. Infect Immun 29:417-424 Bos HJ 1979 Entamoeba histolyrica cytopathogenicity of intact amebae and cell-free extracts: isolation and characterization of an intracellular toxin. Exp Parasitol47:369-377 Bos HJ, Hage AJ 1975 Virulence of bacteria-associated, Crithidia-associated, and axenic Entamoeba Histolytica: experimental hamster liver infections with strains from patients and carriers. - Dongyou Liu(Author)
- 2012(Publication Date)
- CRC Press(Publisher)
cyst. form. of. the. organism. and. in. 1903,. Schaudin. named. it. Entamoeba Histolytica . and. differentiated. it. from. other. Entamoeba .species.[6] . .Leonard.Rogers.designated.emetine. as.the.first.effective.treatment.for.amoebiasis.in.1912.[6] . .In. 1913,.Walker.and.Sellards.conducted.controlled.experiments. with. E. histolytica .on.prisoners.in.the.Philippines.[7] . .They. found.that.transmission.occurred.via.cysts,.not.trophozoites,. asymptomatic.carriers.acted.as.reservoirs.and.were.respon-sible.for.transmission,.there.were.differences.between.indi-viduals’.disease.risk.and.there.were.differences.in.organism. strain.virulence.[7] . .In.1925,.Brumpt.formulated.the.theory. of.the.existence.of.two.distinct.but.morphologically.identical. species,.namely,.the.pathogenic.invasive.form. E. histolytica . and.the.nonpathogenic,.noninvasive.form. E. dispar .[6] . .His. hypothesis.was.dismissed.at.the.time.and.gained.little.sup-port. until. isoenzyme. typing. differentiated. pathogenic. and. nonpathogenic.strains.[8] . .In.1993,.Clark.and.Diamond,.on. Entamoeba Damien Stark and John Ellis CONTENTS 6.1 . Introduction. ....................................................................................................................................................................... 63 6.1.1 . History,.Classification,.and.Morphology. ............................................................................................................... 63 6.1.2 . Entamoeba .Species.That.Infect.Humans. .............................................................................................................. 64 6.1.3 . Life.Cycle.and.Epidemiology. ................................................................................................................................ 64 6.1.4 . Pathogenesis,.Clinical Presentation,.and.Treatment. ..............................................................................................- eBook - PDF
Microbial Foodborne Diseases
Mechanisms of Pathogenesis and Toxin Synthesis
- Jeffrey W. Cary, John E. Linz, Deepak Bhatnagar(Authors)
- 1999(Publication Date)
- CRC Press(Publisher)
Infect. Dis. 171(4):976-983. Guerrant, R. L. (1994). Principles and syndromes of enteric infection, Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, eds., G. L. Mandell, J. E . Bennett and R. Dolin. New York: Churchill Livingstone Inc. pp. 945-962. Guerrant, R. L., Brush, J., Ravdin, J. I., Sullivan, J. A. and Mandell, G. L . 1981. Interaction between Entamoeba Histolytica and leukocytes, J. Infect. Dis. 143(l):83-93. Gut, J. and Nelson, R. D. 1994. Cryptosporidium parvum sporozoites deposit trails of 11A5 antigen during gliding locomotion and shed 11A5 antigen during invasion of MDCK cells in vitro, J. Euk. Microbiol. 41(5):42S. Haas, C. and Rose, J. (1994). Reconciliation of microbial risk models and outbreak epidemiology: the case of the Milwaukee outbreak, Proceedings of the American Water Works Association, 1994. Denver: American Water Works Association, pp. 517-523. Hamann, L., BuB, H. and Tannich, E . 1997. Tetracycline-controlled gene expression in Enta-moeba histolytica, Mol. Biochem. Parasitol. 84(1):83—91. Hamann, L., Nickel, R. and Tannich, E . 1995. Transfection and continuous expression of Entamoeba Histolytica and Cryptosporidium parvum 459 heterologous genes in the protozoan parasite Entamoeba Histolytica, Proc. Natl. Acad. Sci. USA 92(19):8975-8979. Haque, R., Faruque, A. S. G., Hahn, P., Lyerly, D. M. and Petri, W. A. Jr. 1997. Entamoeba Histolytica and Entamoeba dispar infection in children in Bangladesh, J. Infect. Dis. 175(3):734-736. Haque, R., Kress, K., Wood, S., Jackson, T. H. F. G., Lyerly, D., Wilkins, T. and Petri, W. A. Jr. 1993. Diagnosis of pathogenic Entamoeba Histolytica infection using a stool ELISA based on monoclonal antibodies to the galactose-specific adhesin, J. Infect. Dis. 167(l):247-249. Haque, R., Neville, L . M , Hahn, P. and Petri, W. A. Jr. 1995. Rapid diagnosis of Entamoeba infection using the Entamoeba and Entamoeba Histolytica stool antigen detection kits, J.
Index pages curate the most relevant extracts from our library of academic textbooks. They’ve been created using an in-house natural language model (NLM), each adding context and meaning to key research topics.