Chemistry
Electromagnetic Spectrum
The electromagnetic spectrum refers to the entire range of electromagnetic radiation, including radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Each type of radiation has different wavelengths and frequencies, and they are used in various applications such as communication, medical imaging, and spectroscopy. Understanding the electromagnetic spectrum is crucial in studying the interactions of matter with electromagnetic radiation.
Written by Perlego with AI-assistance
Related key terms
1 of 5
12 Key excerpts on "Electromagnetic Spectrum"
- Prakash Singh Bisen, Anjana Sharma(Authors)
- 2012(Publication Date)
- CRC Press(Publisher)
115 5 Spectroscopy 5.1 INTRODUCTION 5.1.1 D EFINITION AND G ENERAL P RINCIPLES As a useful working definition, spectroscopy can be defined as the interaction of electromagnetic radiation (EM) with matter, although this does not include mass spectroscopy. Several factors have led to the branching of spectroscopy in different directions. Most significant is the order of mag-nitude of the energies involved, but additional factors such as the presence of a magnetic field and instrumentation considerations have led to the techniques of ultraviolet (UV), infrared (IR), nuclear magnetic resonance (NMR), and electron spin resonance (ESR) spectroscopy. Breakup and analysis of the above definition will be useful before delving into the details of the different spectroscopic techniques mentioned. The definition includes 1. Electromagnetic radiation 2. Interaction of EM with matter 3. Matter EM radiation consists of an electric field perpendicular to a magnetic field and both at right angles to the direction of propagation of light (Figure 5.1). A fundamental property of EM radiation is that it can behave as though it exists as discrete quanta or packets of energy: E h = υ where E = energy h = Planck’s constant = 6.63 × 10 − 34 J·s υ = frequency of radiation in Hertz There are two ways in which EM radiation interacts with matter: absorption and emission. Absorption occurs when incident radiation increases the energy of a system. An increase in energy is manifested as a decrease in intensity of emergent radiation. Emission occurs when there is a decrease in the energy of a system. Decrease in energy may be due to 1. Thermal energy loss: Energy loss by molecular or submolecular motion like a collision, vibration, or rotation. 2. EM emission: This results in phosphorescence and fluorescence. 3. Photochemical reactions: There is a competition between the three processes, also called relaxation processes.- eBook - PDF
Engineering Chemistry
Fundamentals and Applications
- Shikha Agarwal(Author)
- 2016(Publication Date)
- Cambridge University Press(Publisher)
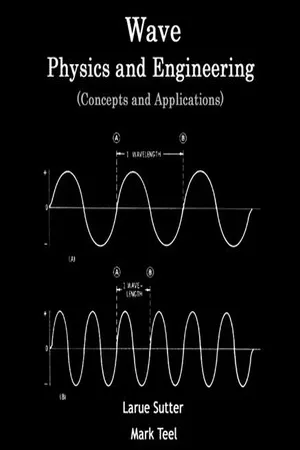
15.1 Introduction Spectroscopy is that branch of science which deals with the study of the interaction of electromagnetic radiation with matter. It is the most powerful tool available for the study of structures of atoms and molecules. Before discussing how electromagnetic radiations interact with matter, it is important to learn about electromagnetic radiations. Electromagnetic radiations Electromagnetic radiation is a form of radiant energy which has both particle as well as wave nature. In vacuum, it normally travels in a straight line with the speed of light (3 ×10 8 m/s). It has both electric and magnetic field components which are coplanar and oscillate perpendicular to each other and perpendicular to the direction of wave propagation (Fig. 15.1). Figure 15.1 Planes of electromagnetic waves Properties of electromagnetic radiations The properties of electromagnetic radiation can be described easily by ascribing wave nature to these radiations. SPECTROSCOPY Chapter 15 816 Engineering Chemistry: Fundamentals and Applications (a) Wavelength It is denoted by l (lambda) and is defined as the distance between two adjacent crests (C–C) or troughs (T–T) in a particular wave (Fig. 15.2). It can be expressed in cm. The other units for expressing wavelength are given below. (i) Angstrom (Å) 1 Å = 10 –8 cm = 10 –10 m (ii) Nanometer (nm) or millimicron (mm ) 1 nm = 1 mm = 10 −7 cm = 10 −9 m (iii) Micron (m) 1m =10 −4 cm = 10 −6 m Figure 15.2 Properties of electromagnetic radiations The wavelength of visible light ranges from 3800 Å (violet end) to 7600 Å (red end). (b) Frequency It is denoted by n (nu) and is defined as the number of waves which can pass through a point in one second. Frequency is expressed in cycles per second or Hertz (Hz) where 1 Hz = 1 cycle/s. Frequency ( ) c ν λ = where c = velocity of electromagnetic radiation in cm/s = 2.998 × 10 10 cm/s. l = wavelength. We know that the wavelength of visible light is 3800−7600 Å. - No longer available |Learn more
- (Author)
- 2014(Publication Date)
- Learning Press(Publisher)
________________________ WORLD TECHNOLOGIES ________________________ Chapter- 6 Electromagnetic Spectrum The Electromagnetic Spectrum is the range of all possible frequencies of electro-mag-netic radiation. The Electromagnetic Spectrum of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object. The Electromagnetic Spectrum extends from low frequencies used for modern radio to gamma radiation at the short-wavelength end, covering wavelengths from thousands of kilometers down to a fraction of the size of an atom. The long wavelength limit is the size of the universe itself, while it is thought that the short wavelength limit is in the vicinity of the Planck length, although in principle the spectrum is infinite and continuous. Although some radiations are marked as N for no in the diagram, some waves do in fact penetrate the atmosphere, although extremely minimally compared to the other radiations. ________________________ WORLD TECHNOLOGIES ________________________ Legend γ= Gamma rays MIR= Mid infrared HF= High freq. HX= Hard X-rays FIR= Far infrared MF= Medium freq. SX= Soft X-rays Radio waves LF= Low freq. EUV= Extreme ultraviolet EHF= Extremely high freq. VLF= Very low freq. NUV= Near ultraviolet SHF= Super high freq. VF/ULF= Voice freq. Visible light UHF= Ultra high freq. SLF= Super low freq. NIR= Near Infrared VHF= Very high freq. ELF= Extremely low freq. Freq=Frequency Range of the spectrum EM waves are typically described by any of the following three physical properties: the frequency f , wavelength λ, or photon energy E . Frequencies range from 2.4×10 23 Hz (1 GeV gamma rays) down to the local plasma frequency of the ionized interstellar medium (~1 kHz). Wavelength is inversely proportional to the wave frequency, so gamma rays have very short wavelengths that are fractions of the size of atoms, whereas wavelengths can be as long as the universe. - No longer available |Learn more
- (Author)
- 2014(Publication Date)
- Academic Studio(Publisher)
A common laboratory spectroscope can detect wavelengths from 2 nm to 2500 nm. Detailed information about the physical properties of objects, gases, or even stars can be obtained from this type of device. Spectroscopes are widely used in astrophysics. For example, many hydrogen atoms emit a radio wave photon which has a wavelength of 21.12 cm. Also, frequencies of 30 Hz and below can be produced by and are important in the study of certain stellar nebulae and frequencies as high as 2.9×10 27 Hz have been detected from astrophysical sources. Rationale Electromagnetic radiation interacts with matter in different ways in different parts of the spectrum. The types of interaction can be so different that it seems to be justified to refer to different types of radiation. At the same time, there is a continuum containing all these different kinds of electromagnetic radiation. Thus we refer to a spectrum, but divide it up based on the different interactions with matter. Region of the spectrum Main interactions with matter Radio Collective oscillation of charge carriers in bulk material (plasma oscillation). An example would be the oscillation of the electrons in an antenna. Microwave Plasma oscillation, molecular rotation ________________________ WORLD TECHNOLOGIES ________________________ through far infrared Near infrared Molecular vibration, plasma oscillation (in metals only) Visible Molecular electron excitation (including pigment molecules found in the human retina), plasma oscillations (in metals only) Ultraviolet Excitation of molecular and atomic valence electrons, including ejection of the electrons (photoelectric effect) X-rays Excitation and ejection of core atomic electrons, Compton scattering (for low atomic numbers) Gamma rays Energetic ejection of core electrons in heavy elements, Compton scattering (for all atomic numbers), excitation of atomic nuclei, including dissociation of nuclei High energy gamma rays Creation of particle-antiparticle pairs. - eBook - PDF
- James W. Robinson, Eileen Skelly Frame, George M. Frame II(Authors)
- 2014(Publication Date)
- CRC Press(Publisher)
The visible light region is expanded to show the colors associated with wavelength ranges. 64 UNDERGRADUATE INSTRUMENTAL ANALYSIS 2.1.2 How Does Electromagnetic Radiation Interact with Matter? Spectroscopy.is.the.study.of.the.interaction.of.radiant.energy.(light).with.matter . .We.know. from.quantum.mechanics.that.energy.is.really.just.a.form.of.matter.and.that.all.matter.exhibits.the. properties.of.both.waves.and.particles . .However,.matter.composed.of.molecules,.atoms,.or.ions,. which.exists.as.solid.or.liquid.or.gas,.exhibits.primarily.the.properties.of.particles . .Spectroscopy. studies.the.interaction.of.light.with.matter.defined.as.materials.composed.of.molecules.or.atoms. or.ions . In.a.gas,.atoms.or.molecules.are.widely.separated.from.each.other;.in.liquids.and.solids,.the. atoms.or.molecules.are.closely.associated . .In.solids,.the.atoms.or.molecules.may.be.arranged.in. a.highly.ordered.array,.called.a. crystal ,.as.they.are.in.many.minerals,.or.they.may.be.randomly. arranged,.or. amorphous ,.as.they.are.in.many.plastics . .Atoms,.molecules,.and.ions.are.in.constant. motion. whatever. their. physical. state. or. arrangement . . For. molecules,. many. types. of. motion. are. involved. .Molecules.can.rotate,.vibrate,.and.translate.(move.from.place.to.place.in.space) . .Interaction. with.radiant.energy.can.affect.these.molecular.motions . .Molecules.that.absorb.IR.radiation.vibrate. with.greater.amplitude;.interaction.with.UV/VIS.light.can.move.bonding.electrons.to.higher.energy. levels.in.molecules . .A.change.in.any.form.of.motion.or.electron.energy.level.involves.a.change.in. the.energy.of.the.molecule . .Such.a.change.in.energy.is.called.a. transition ;.we.have.the.possibility.of. vibrational.transitions,.rotational.transitions,.and.electronic.transitions.in.molecules . .We.have.some. of.the.same.kinds.of.motion.in.atoms.and.ions . - No longer available |Learn more
- (Author)
- 2014(Publication Date)
- Academic Studio(Publisher)
A common laboratory spectroscope can detect wavelengths from 2 nm to 2500 nm. Detailed information about the physical properties of objects, gases, or even stars can be obtained from this type of device. It is widely used in astrophysics. For example, hydrogen atoms emit radio waves of wavelength 21.12 cm. Soundwaves are not electromagnetic radiation. At the lower end of the electro-magnetic spectrum, about 20 Hz to about 20kHz, are frequencies that might be considered in the audio range, however, electromagnetic waves cannot be directly perceived by human ears. Sound waves are the osscilating compression of molecules. To be heard, electromagnetic radiation must be converted to air pressure waves, or if the ear is submerged, water pressure waves. Light EM radiation with a wavelength between approximately 400 nm and 700 nm is directly detected by the human eye and perceived as visible light. Other wavelengths, especially nearby infrared (longer than 700 nm) and ultraviolet (shorter than 400 nm) are also sometimes referred to as light, especially when visibility to humans is not relevant. If radiation having a frequency in the visible region of the EM spectrum reflects off of an object, say, a bowl of fruit, and then strikes our eyes, this results in our visual perception of the scene. Our brain's visual system processes the multitude of reflected frequencies into different shades and hues, and through this not-entirely-understood psychophysical phenomenon, most people perceive a bowl of fruit. ________________________ WORLD TECHNOLOGIES ________________________ At most wavelengths, however, the information carried by electromagnetic radiation is not directly detected by human senses. Natural sources produce EM radiation across the spectrum, and our technology can also manipulate a broad range of wavelengths. Optical fiber transmits light which, although not suitable for direct viewing, can carry data that can be translated into sound or an image. - No longer available |Learn more
- (Author)
- 2014(Publication Date)
- Library Press(Publisher)
A common laboratory spectroscope can detect wavelengths from 2 nm to 2500 nm. Detailed information about the physical properties of objects, gases, or even stars can be obtained from this type of device. It is widely used in astrophysics. For example, hydrogen atoms emit radio waves of wavelength 21.12 cm. Soundwaves are not electromagnetic radiation. At the lower end of the electro-magnetic spectrum, about 20 Hz to about 20kHz, are frequencies that might be considered in the audio range, however, electromagnetic waves cannot be directly perceived by human ears. Sound waves are the osscilating compression of molecules. To be heard, electromagnetic radiation must be converted to air pressure waves, or if the ear is submerged, water pressure waves. Light EM radiation with a wavelength between approximately 400 nm and 700 nm is directly detected by the human eye and perceived as visible light. Other wavelengths, especially nearby infrared (longer than 700 nm) and ultraviolet (shorter than 400 nm) are also sometimes referred to as light, especially when visibility to humans is not relevant. If radiation having a frequency in the visible region of the EM spectrum reflects off of an object, say, a bowl of fruit, and then strikes our eyes, this results in our visual perception of the scene. Our brain's visual system processes the multitude of reflected frequencies into different shades and hues, and through this not-entirely-understood psychophysical phenomenon, most people perceive a bowl of fruit. ____________________ WORLD TECHNOLOGIES ____________________ At most wavelengths, however, the information carried by electromagnetic radiation is not directly detected by human senses. Natural sources produce EM radiation across the spectrum, and our technology can also manipulate a broad range of wavelengths. Optical fiber transmits light which, although not suitable for direct viewing, can carry data that can be translated into sound or an image. - No longer available |Learn more
- (Author)
- 2014(Publication Date)
- Learning Press(Publisher)
A common laboratory spectroscope can detect wavelengths from 2 nm to 2500 nm. Detailed information about the physical properties of objects, gases, or even stars can be obtained from this type of device. It is widely used in astrophysics. For example, hydrogen atoms emit radio waves of wavelength 21.12 cm. Soundwaves are not electromagnetic radiation. At the lower end of the electroma-gnetic spectrum, about 20 Hz to about 20kHz, are frequencies that might be considered in the audio range, however, electromagnetic waves cannot be directly perceived by human ears. Sound waves are the osscilating compression of molecules. To be heard, electromagnetic radiation must be converted to air pressure waves, or if the ear is submerged, water pressure waves. Light EM radiation with a wavelength between approximately 400 nm and 700 nm is directly detected by the human eye and perceived as visible light. Other wavelengths, especially nearby infrared (longer than 700 nm) and ultraviolet (shorter than 400 nm) are also sometimes referred to as light, especially when visibility to humans is not relevant. If radiation having a frequency in the visible region of the EM spectrum reflects off of an object, say, a bowl of fruit, and then strikes our eyes, this results in our visual perception of the scene. Our brain's visual system processes the multitude of reflected frequencies into different shades and hues, and through this not-entirely-understood psychophysical phenomenon, most people perceive a bowl of fruit. ____________________ WORLD TECHNOLOGIES ____________________ At most wavelengths, however, the information carried by electromagnetic radiation is not directly detected by human senses. Natural sources produce EM radiation across the spectrum, and our technology can also manipulate a broad range of wavelengths. Optical fiber transmits light which, although not suitable for direct viewing, can carry data that can be translated into sound or an image. - eBook - PDF
- Khetarpaul, Neelam(Authors)
- 2021(Publication Date)
- Daya Publishing House(Publisher)
A spectrum is a plot of the intensity of energy detected versus the wavelength (or mass or momentum or frequency, etc.) of the energy. A spectrum can be used to obtain information about atomic and molecular energy levels, molecular geometries, chemical bonds, interactions of molecules, and related processes. Often, spectra are used to identify the components of a sample (qualitative analysis). Spectra may also be used to measure the amount of material in a sample (quantitative analysis). This ebook is exclusively for this university only. Cannot be resold/distributed. Spectroscopy/spectrometry is often used in physical and analytical chemistry for the identification of substances through the spectrum emitted from or absorbed by them. It is also heavily used in astronomy and remote sensing. Classification of Methods of Spectroscopy It generally depends upon: 1.Nature of excitation measured 2.Measurement process Nature of Excitation Measured The type of spectroscopy depends on the physical quantity measured. Normally, the quantity that is measured is an intensity, either of energy absorbed or produced. PElectromagnetic spectroscopy involves interactions of matter with electromagnetic radiation, such as light. PElectron spectroscopy involves interactions with electron beams. Auger spectroscopy involves inducing the Auger effect with an electron beam. In this case the measurement typically involves the kinetic energy of the electron as variable. PAcoustic spectroscopy involves the frequency of sound. PDielectric spectroscopy involves the frequency of an external electrical field. PMechanical spectroscopy involves the frequency of an external mechanical stress, e.g. a torsion applied to a piece of material. Measurement Process Most spectroscopic methods are differentiated as either atomic or molecular based on whether or not they apply to atoms or molecules. - eBook - PDF
- Harry G. Brittain(Author)
- 2006(Publication Date)
- CRC Press(Publisher)
CLASSICAL DESCRIPTION OF ELECTROMAGNETIC RADIATION In the classical world, a molecule had to contain either a permanent or transient dipole moment in order to interact with electromagnetic radiation. A bond Electromagnetic Radiation and Spectroscopy 3 Table 1 Spectral Regions of Electromagnetic Radiation Electromagnetic radiation type Wavelength (m) Wavenumber (cm 2 1 ) Frequency (Hz) g -ray Less than 1.0 10 2 10 Greater than 100,000,000 Greater than 3.0 10 18 X-Ray 1.0 10 2 10 to 1.0 10 2 8 1,000,000 to 100,000,000 3.0 10 16 to 3.0 10 18 Ultraviolet Far 1.0 10 2 8 to 2.0 10 2 7 (10 to 200 nm) 50,000 to 1,000,000 1.5 10 15 to 3.0 10 16 Near 2.0 10 2 7 to 4.0 10 2 7 (200 to 400 nm) 25,000 to 50,000 7.5 10 14 to 1.5 10 15 Visible 4.0 10 2 7 to 7.5 10 2 7 (400 to 750 nm) 13,350 to 25,000 4.0 10 14 to 7.5 10 14 Infrared Near 7.5 10 2 7 to 2.5 10 2 6 (0.75 to 2.5 m m) 4,000 to 13,350 1.2 10 14 to 4.0 10 14 Mid 2.5 10 2 6 to 2.5 10 2 5 (2.5 to 25 m m) 400 to 4000 1.2 10 13 to 1.2 10 14 Far 2.5 10 2 5 to 4.0 10 2 4 (25 to 400 m m) 25 to 400 7.5 10 11 to 1.2 10 13 Microwave 4.0 10 2 4 to 1.0 10 0 (0.04 to 100 cm) 0.01 to 25 3.0 10 8 to 7.5 10 11 Radiowave Greater than 1 Less than 0.01 Less than 3.0 10 8 4 Brittain formed between atoms having different electronegativity values results in a charge separation and in the formation of a permanent electric dipole. The total electric moment, P , of a molecule involves a summation of charge separations over all the electronic and nuclear coordinates: P ¼ X i q i r i (5) where q i defines the magnitude of charges separated by a distance r i . To a first approximation, one can assume that the nuclear coordinates are fixed and that inner-shell electrons have a spherical charge distribution. Therefore, the major contribution to the total electric moment arises from the electronic cloud of the bonding and nonbonding valence electrons. - eBook - PDF
- William H. Brown, Thomas Poon(Authors)
- 2017(Publication Date)
- Wiley(Publisher)
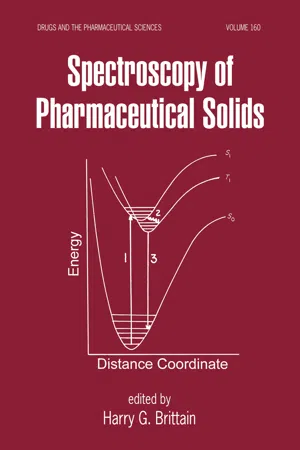
We will see that molecules interact with different forms of electromagnetic radiation by absorbing their energy in various ways 344 C H A P T E R 11 Spectroscopy 11.2 What Is Molecular Spectroscopy? Organic molecules are flexible structures. They rotate in solution, their bonds stretch, bend, and rotate, and they contain electrons that can move from one electronic energy level to another. We know from experimental observations and from theories of molecular structure that all energy changes within a molecule are quantized; that is, they are subdi- vided into small, but well‐defined, increments. For example, vibrations of bonds within molecules can undergo transitions only between allowed vibrational energy levels. We can cause an atom or molecule to undergo a transition from energy state E 1 to a higher energy state E 2 by irradiating it with electromagnetic radiation corresponding to the energy difference between states E 1 and E 2 , as illustrated schematically in Figure 11.1. When the atom or molecule returns to the ground state E 1 , an equivalent amount of energy is emitted. Energy E 2 E 2 E 1 E 1 Atom or molecule in energy state E 1 Absorption of energy Atom or molecule in energy state E 2 Energy in the form of electromagnetic radiation E the energy of the radiation must be equal to the energy difference ( E ) between E 2 and E 1 . Any amount greater or smaller than this difference will not result in this transition FIGURE 11.1 Absorption of energy in the form of electromagnetic radiation excites an atom or a molecule in energy state E 1 to a higher energy state E 2 . Molecular spectroscopy is the experimental process of measuring which frequencies of radiation a substance absorbs or emits and then correlating those frequencies with specific types of molecular structures. - eBook - PDF
- Andrew Fraknoi, David Morrison, Sidney C. Wolff(Authors)
- 2016(Publication Date)
- Openstax(Publisher)
Table 5.1 summarizes the bands of the Electromagnetic Spectrum and indicates the temperatures and typical astronomical objects that emit each kind of electromagnetic radiation. While at first, some of the types of radiation listed in the table may seem unfamiliar, you will get to know them better as your astronomy course continues. You can return to this table as you learn more about the types of objects astronomers study. Types of Electromagnetic Radiation Type of Radiation Wavelength Range (nm) Radiated by Objects at This Temperature Typical Sources Gamma rays Less than 0.01 More than 10 8 K Produced in nuclear reactions; require very high-energy processes X-rays 0.01–20 10 6 –10 8 K Gas in clusters of galaxies, supernova remnants, solar corona Ultraviolet 20–400 10 4 –10 6 K Supernova remnants, very hot stars Visible 400–700 10 3 –10 4 K Stars Infrared 10 3 –10 6 10–10 3 K Cool clouds of dust and gas, planets, moons Microwave 10 6 –10 9 Less than 10 K Active galaxies, pulsars, cosmic background radiation Radio More than 10 9 Less than 10 K Supernova remnants, pulsars, cold gas Table 5.1 156 Chapter 5 Radiation and Spectra This OpenStax book is available for free at http://cnx.org/content/col11992/1.13 Radiation and Temperature Some astronomical objects emit mostly infrared radiation, others mostly visible light, and still others mostly ultraviolet radiation. What determines the type of electromagnetic radiation emitted by the Sun, stars, and other dense astronomical objects? The answer often turns out to be their temperature. At the microscopic level, everything in nature is in motion. A solid is composed of molecules and atoms in continuous vibration: they move back and forth in place, but their motion is much too small for our eyes to make out. A gas consists of atoms and/or molecules that are flying about freely at high speed, continually bumping into one another and bombarding the surrounding matter.
Index pages curate the most relevant extracts from our library of academic textbooks. They’ve been created using an in-house natural language model (NLM), each adding context and meaning to key research topics.