Geography
Heavy Metals
Heavy metals are metallic elements with high atomic weights and densities, such as lead, mercury, and cadmium. They are naturally occurring in the Earth's crust but can also be released into the environment through human activities like mining and industrial processes. Heavy metals can accumulate in soil and water, posing risks to ecosystems and human health.
Written by Perlego with AI-assistance
Related key terms
1 of 5
12 Key excerpts on "Heavy Metals"
- eBook - PDF
- Eric C. Brevik, Lynn C. Burgess, Eric C. Brevik, Lynn C. Burgess(Authors)
- 2012(Publication Date)
- CRC Press(Publisher)
In the final section, three case studies are presented. The focus of the chapter is on toxicity-related health problems resulting from environmental pollution, but it should be recognized that human health is also affected by natural soil deficiencies of some of the metals (Brevik, 2009; Oliver, 1997). 3.1.2 W HAT I S A H EAVY M ETAL ? Broadly, the term heavy metal is usually applied to those elements with metallic properties and a density of 5 g/cm 3 or higher. However, as van der Perk (2006, p. 125) notes: “... the term heavy met-als has no sound terminological or scientific basis.” For example, the criteria for defining “heavy” can vary between authors, and not all the Heavy Metals commonly discussed act as metals in the generally accepted sense; some are classed as semimetals or metalloids (e.g., arsenic and antimony) (Kabata-Pendias and Mukherjee, 2007; Dufuss, 2002). Moreover, some researchers prefer to use the alternative term trace elements . However, as Alloway (1995, p. 3) comments, heavy metal is the “most widely recognised and used term” for a large group of trace elements that are “both industri-ally and biologically important.” Opinions also differ as to which elements make up that large group of trace elements, but for the purpose of this chapter the practical approach advocated by Fergusson (1990) seems apt. He focuses on those elements that are causing the most problems for large numbers of people, either as direct threats to health, or indirectly, by causing environmental changes that have health consequences for people. On this basis, the specific elements considered in this chapter are arsenic * (As), lead (Pb), cadmium (Cd), chromium (Cr), copper (Cu), mercury (Hg), nickel (Ni), and zinc (Zn). 3.1.3 S OURCES 3.1.3.1 Natural Sources and Biological Functions Heavy Metals are constituents of the rocks that make up Earth’s surface. - eBook - PDF
- Gautam, Ashutosh(Authors)
- 2021(Publication Date)
- Daya Publishing House(Publisher)
Therefore, Any toxic metal may be called heavy metal, irrespective of their atomic mass or density (Singh, 2007). The most important thing is their (Heavy Metals) impact on biosphere. Heavy Metals are natural components of the Earth’s crust. They cannot be degraded or destroyed completely. To a small extent they enter our bodies via food, drinking water and air. As trace elements, some Heavy Metals (e.g. copper, selenium, zinc) are essential to maintain the metabolism of the human body. However, at higher concentrations they can lead to poisoning. Heavy metal poisoning could result; for instance, from drinking-water contamination e.g., lead pipes, high ambient air concentrations near emission sources, or intake via the food chain (Lenntech, 2016), but indiscriminate human activities have drastically altered their geochemical cycles and biochemical balance. This results in accumulation of metals in plant parts having secondary metabolites, which is responsible for a particular pharmacological activity. Prolonged exposure to Heavy Metals such as cadmium, copper, lead, nickel, and zinc can cause deleterious health effects in humans (Singh et al., 2011). Heavy Metals are considered as hazardous because they tend to bioaccumulate (Accumulation in an organism). Heavy Metals can enter a water supply by industrial and consumer waste, or even from acidic rain breaking down soils and releasing Heavy Metals into streams, lakes, rivers, and groundwater (Lenntech, 2016). This ebook is exclusively for this university only. Cannot be resold/distributed. Heavy Metals Contamination and its Impact on Human and Environmental Health 127 Sources of Heavy Metal in the Environment Heavy Metals occur naturally in the environment from pedogenetic processes of weathering of parent materials and also through anthropogenic sources. - eBook - PDF
- Kumar, Arvind(Authors)
- 2021(Publication Date)
- Daya Publishing House(Publisher)
Because of their non-biodegradable nature, Heavy Metals accumulated by organisms undergo biomedical cycle in the environment during which they are transformed into various chemical species. Of particular importance, all types of natural water sources like river, lakes, groundwater and sea as well as the atmosphere (rain and snow) for transfer of metals through the environment. The observed environmental degradation and behavioural disturbances that occur along with other various human ailments are of great concern. Deadly Heavy Metals The most hazardous and important Heavy Metals are lead, mercury, This ebook is exclusively for this university only. Cannot be resold/distributed. cadmium, arsenic, thallium and selenium. These metals have a history as occupational hazards and some of them have been linked to cancer and heart diseases; accordingly, they belong to the class of first-order priority in eco-toxicology. Some metals that are essential nutrients, e.g. copper, zinc, tin etc. can exert toxic action also, depending on their concentration levels. For these metals, a narrow ‘concentration window’ exists between the toxic and essential levels. Largely their physiological forms control the potential toxicity of various metals ( Table 1.2 ). Environmental degradation with metals is due to their release into environment during manufacture of goods, industrial treatments like galvanizing, as by-products or the discharge as waste, both as effluents or solids. Arsenic, lead, zinc and cadmium were detected in the galvanizing plant effluents (Pandey and Seth, 1985). Metals may occur in the environment as hydrated ionic species or they may form a variety of complexes with inorganic and organic ligands. In water, they occur as complexes and diverse mixture of soluble and insoluble forms such as ionic species, inorganic and organic complexes and/or associated with colloids and suspended particulate matter. - eBook - ePub
Biological Inorganic Chemistry
A New Introduction to Molecular Structure and Function
- Robert R. Crichton(Author)
- 2012(Publication Date)
- Elsevier(Publisher)
Chapter 23
Metals in the Environment
Introduction Environmental Pollution and Heavy Metals Aluminium Cadmium Mercury Lead Metals as PoisonsIntroduction Environmental Pollution and Heavy Metals
We have already seen in earlier chapters that even essential metal ions can be toxic – as the historical father of toxicology, Paracelsus (1493–1541), wrote“Alle Ding’ sind Gift, und nichts ohn’ Gift; allein die Dosis macht, daß ein Ding kein Gift ist. ”“Everything is poisonous, and nothing is not; only the dose ensures that something is not poisonous. ”This dictum that “The dose makes the poison” is as true today as when it was stated five centuries ago. But it is also applicable to a number of metals which we encounter in our constantly evolving environment, many of which have no biological ‘raison d’être1 ’. These include a number of toxic metals which we have introduced into our environment in the course of the industrialisation of our society. Over the past decades, there has been an increasing awareness throughout the world of the health and developmental risks associated with environmental exposure to “Heavy Metals2 ”, such as lead (Pb), mercury (Hg), and cadmium (Cd). This term has been widely used, often to describe a group of metals and metalloids that have been associated with contamination and potential toxicity or ecotoxicity. At the same time, legal regulations often specify a list of “Heavy Metals” to which they apply. Such lists differ from one set of regulations to another and the term is sometimes used without even specifying which “Heavy Metals” are covered. However, there is no authoritative definition to be found in the relevant literature. And while we could agree that all of those mentioned above fall into the category of Heavy Metals that have been released into the environment by the activity of man, where do we place aluminium, the insidious pollution of which has crept upon us notably as a consequence of acid rain, as we pointed out in Chapter 1 - eBook - ePub
Ecology of Estuaries
Anthropogenic Effects
- Michael J. Kennish(Author)
- 2019(Publication Date)
- CRC Press(Publisher)
5 Heavy MetalsIntroduction
The continuous increase in heavy metal contamination of estuarine and coastal marine waters is directly attributable to industrialization and development in the coastal zone. The contamination by most Heavy Metals in coastal environments reflects localized impacts from point or multipoint discharges from municipal and industrial sources.1 Because of their persistence in the environment, their toxicity at high concentrations, and their tendency to accumulate in the tissues of biota, Heavy Metals pose potentially hazardous conditions for man. Hence, they have been the subject of ever-expanding research activities to control their concentrations in estuarine and coastal marine habitats.Heavy Metals, as defined by Viarengo,2 are a group of elements with atomic weights ranging from 63.546 to 200.590 and are characterized by similar electronic distribution in the external shell (e.g., Cu, Zn, Cd, and Hg). These exclude the alkaline earth metals, alkali metals, lanthanides, and actinides.3 Although these elements are toxic to estuarine and marine organisms above a threshold availability, many of them are essential to metabolism at lower concentrations.4 Necessary trace elements for life processes include (but are not limited to) cobalt, copper, iron, manganese, molybdenum, vanadium, strontium, and zinc;5 , 6 however, any of these elements can be toxic to organisms when present in high concentrations.7 Of great concern as potential environmental contaminants are cadmium, chromium, mercury, lead, selenium, arsenic, and antimony, which have contributed to severe insidious pollution problems in various estuarine and coastal ecosystems of the U.S. Some Heavy Metals, such as cadmium and lead, have no known biological function,3 - eBook - PDF
- Leo M.L. Nollet, Leen S. P. De Gelder, Leo M.L. Nollet, Leen S. P. De Gelder(Authors)
- 2013(Publication Date)
- CRC Press(Publisher)
385 © 2011 Taylor & Francis Group, LLC 15 Heavy Metals, Major Metals, Trace Elements Jorge E. Marcovecchio, Sandra E. Botté, Claudia E. Domini, and Rubén H. Freije The contamination of natural waters is a worldwide distributed problem which deserves large attention not only due to its environmental hazardous effects but also for the risks involved to human health as well as economic damages it produces. Between the wide diversity of pollutants affecting water resources, Heavy Metals receive particular concern considering their strong toxicity even at low concentrations. The occurrence of Heavy Metals in water bodies can be of natural origin (i.e., eroded minerals within sediments, leaching of ore deposits, and vulcanism extruded products) or anthropogenic in nature (i.e., solid waste disposal, industrial or domestic effluents, harbor channels dredging). The term heavy metal includes both essential and nonessential trace metals, which may be toxic to the organisms depending on their own properties, availability (chemical speciation), and concentration levels. Heavy Metals (Ag, As, Cd, Cu, Cr, Hg, Ni, Pb, Zn) can be present in the aquatic system in both dis-solved forms (which can cause toxic effects on a wide diversity of organisms, including vertebrates) and particulated ones (including adsorbed on sediments, suspended particulate matter or colloids, in transi-tional complexes, and Fe/Mn hydroxides nets, linked to organic matter and carbonates, etc.). The dynam-ics which regulates the transference of Heavy Metals between the dissolved and the particulated phases (in both senses) depends on the pH and oxide-reduction potential of the system. Also these parameters regulate the chemical speciation of Heavy Metals within the system. CONTENTS 15.1 Sampling ...................................................................................................................................... - eBook - PDF
- Hosam El-Din M. Saleh, Refaat F. Aglan, Hosam El-Din M. Saleh, Refaat F. Aglan(Authors)
- 2018(Publication Date)
- IntechOpen(Publisher)
They are able to migrate and accumulate in the appropriate barriers. Their concentrations exceed the normal values typical of the natural landscapes in the Absheron peninsula [5]. There is a tendency of the growth of Heavy Metals concentration both in the bottom sediments and in some hydrobionts in the Absheron territory. As a result of contamination by wastewater from various anthropogenic sources including offshore oil fields, Heavy Metals penetrate into the coastal line, natural lakes, and other reservoirs. Usually, the most commonly encountered metals in polluted areas are lead (Pb), chromium (Cr), mercury (Hg), arsenic (As), zinc (Zn), cadmium (Cd), copper (Cu), and nickel (Ni). It is well known that Heavy Metals are nondegradable and persistent pollutants and accumulate in the environment for a long time. In soil, they could be adsorbed and gathered in different parts of plants through root systems. It has been revealed that heavy metal pollution causes adverse effects on the quality and yield of agricultural plants, resulting in the changes of the number, composition, and activity of microorganisms. Practice shows that the geochemical cycle of Heavy Metals under anthropogenic impacts represents serious, at times unpredictable, envi-ronmental effects. As mentioned earlier, due to their migration and accumulation in the envi -ronment, most Heavy Metals can easily enter the food chain and create serious threat to human health. Many Heavy Metals have a strong affinity for sulfur and disturb the enzyme function Oil fields Metals (%) Fe Ni Cr Zn Ba V Co Hg Ag Au La Sb Sc Hf Se × 10 −4 × 10 −5 × 10 −6 × 10 −7 Asphaltenes 1. 600 57 96 750 53 151 110 nd * 289 140 87 300 1500 28 47 2. 138 58 174 270 265 260 64 160 nd 8 28 19 20 26 32 3. 400 63 68 110 540 142 35 570 nd 6 nd 4 13 14 nd Tars 4. 170 8 20 80 5 37 2 14 10 150 4 2 10 23 75 5. 34 23 12 3 nd 62 10 nd 2.4 6 nd nd 2 52 60 6. - eBook - ePub
Heavy Metals in Plants
Physiological to Molecular Approach
- Jitendra Kumar, Shweta Gaur, Prabhat Kumar Srivastava, Rohit Kumar Mishra, Sheo Mohan Prasad, Devendra Kumar Chauhan, Jitendra Kumar, Shweta Gaur, Prabhat Kumar Srivastava, Rohit Kumar Mishra, Sheo Mohan Prasad, Devendra Kumar Chauhan(Authors)
- 2022(Publication Date)
- CRC Press(Publisher)
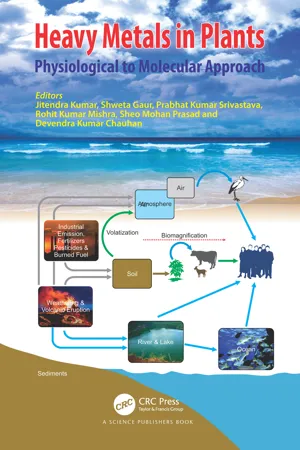
Masindi and Muedi, 2018 ). Heavy Metals are largely present in soil and aquatic ecosystems. Some of the metals called micronutrients (Mn, Co, Cr, Cu, Mo, Se, Fe, and Zn) are essential for plant growth at very low concentrations while macronutrients (Na, Mg, Ca, P, and S) are required in a large amount. While, some metals (Ni, As, Pb, Cd, and Hg) are toxic even in a trace amount. High metal concentration in the environment is hazardous to ecosystems due to its toxic nature, long-term persistence, and bioaccumulation (Conceiçao et al., 2012). The successive transfer of the Heavy Metals in the aquatic and terrestrial food web is hazardous for the residing organism and human health. The metals generate reactive oxygen species like superoxide anion, hydroxyl radical, singlet oxygen, etc., that induce oxidative stress by damaging different biomolecules like DNA, protein, lipids and, bio-membranes, etc. This stress induces inflammation that leads to the development of different cardiovascular, neurodegenerative, and other chronic diseases. In this chapter, there is a comprehensive description of the effects of different Heavy Metals on various ecosystems, crop productivity, and human health.2. Sources of metal contamination
Metals enter into the ecosystem via natural as well as man-made activities (Fig. 1 ). Natural phenomena, i.e., weathering of rocks and volcanic eruptions are the major cause of metal pollution (Masindi and Muedi, 2018 ; Shallari et al., 1998 ). Environmental contamination also occurs through several anthropogenic sources viz; petroleum combustion, power plants, refineries, industries, and use of metals in fertilizers (Arruti et al., 2010 ). Heavy Metals reach the soil through the use of phosphate fertilizers. In the process of extracting phosphate fertilizers from phosphate rock, known as acidulation, different Heavy Metals are produced as a by-product (Mortvedt, 1996 ). The application of fertilizers in agricultural soils not only contaminates the soil but also leaches into groundwater and eventually contaminates it (Dissanayake and Chandrajith, 2009 ). Vehicular traffic is one of the key contributors of metal pollution among different man-made sources (Ferretti et al., 1995 - Prasad, D(Authors)
- 2021(Publication Date)
- Biotech(Publisher)
Heavy Metals may chemically or physically interact with the natural compounds, which changes their forms of existence in the environment. In general they may react with particular species, change oxidation states and precipitate. Heavy Metals may be bound or sorbed by particular natural substances, which may increase or decrease mobility1. The transport mechanisms of Heavy Metals through soil has long presented great interest to both environment and soil scientists because of the possibility of ground water contamination through metal leaching. In general, many soils contain a wide range of Heavy Metals with varying concentration ranges depending on the surrounding geological environment and anthropogenic and natural activities occurring or once occurred. These metals can be Fe (Iron), Cr (Chromium), Mn (Manganese), Ni (Nickel), Zn (Zinc), Cu (Copper), Pb (Lead), Cd (Cadmium), Hg (Mercury), etc. Metal transport is not only dependent on the physiochemical properties of the metals but mostly on the physical and chemical properties of the soil, for example soil organic matter content, clay fraction content, mineralogical composition, pH and more, all of which collectively determine the binding ability of the soil. The properties of the soil may change due to climate change but mostly due to anthropogenic impact. The influence of acid rains on the soils and sorption properties of soil complex has been extensively studied by the scientists from various disciplines. In almost all cases, they found that the acid rains decrease the ability of binding Heavy Metals to soil particles (Dube et al. , 2001). The most common metals, which are found in the soil are lead and cadmium. Lead a heavy, bluish-grey chemical element, is one of the world’s oldest known metals. Its chemical symbol, Pb, comes from the Latin word for lead, plumbum. People have used lead for thousands of years as a building material, for water piping, and to make pottery and other objects.- eBook - PDF
Discharge of Sewage from Sea Outfalls
Proceedings of an International Symposium Held at Church House, London, 27 August to 2 September 1974
- A. L. H. Gameson(Author)
- 2015(Publication Date)
- Pergamon(Publisher)
Paper No. 12 Heavy Metals in the Marine Environment A. Jernelöv Swedish Water and Air Pollution Research Laboratory, Stockholm Some of the Heavy Metals, especially mercury and lead, undoubtedly belong to those that have been most extensively studied as marine pollutants. Despite this it is evident, when making an attempt to summarize present knowledge in the field, that existing data form scattered bits and pieces and that the assumptions and hypotheses required to connect and bring the pieces together are at best unverified. Frequently the data contradict each other and little objective information exists that can be used to confirm or refute the results of other investigations. In the present paper few individual figures for metal concentrations are quoted, but some examples are given to indicate typical values or ranges, or to provide bases for mass-balance calculations. Naturally attempts are made to identify and find patterns in distribution and occurrence. The term 'Heavy Metals' has been used in a very wide (in fact in a non-chemical) sense, and includes metalloids. The information is presented as case studies. Mercury The first incident that drew attention to mercury as an environmental pollutant occurred in the coastal marine environment of Minamata Bay, Japan. Although this tragic event had the positive consequence that we became aware of the hazards that could be connected with mercury pollution, we now know that from most points of view it was not typical of the marine mercury contamination problems. The Minamata case was very localized, methyl mercury being discharged directly to a river and the bay. Around the coasts of the world we have many areas with local mercury pollution, but in most cases mercury is or has been discharged in inorganic form or as organo-mercurials other than methyl mercury. This means that before the mercury becomes a hazardous contaminant in fish or shellfish, it has to undergo methylation. - eBook - PDF
- Waldemar M. Dabrowski, Zdzislaw E. Sikorski(Authors)
- 2004(Publication Date)
- CRC Press(Publisher)
Determination of the roles of microelements and the human daily requirements can be very difficult due to their low concentrations in the human body and problems connected with the elimination of their constant inflow. Throughout the evolution process, the human body developed mechanisms to regulate the absorption of microelements and balance their levels within required ranges. Therefore, human bodies are adjusted to the natural levels at which those elements are present in the non-polluted environment and in non-contaminated foodstuffs. However, human industrial and economic activities are frequently and widely disturbing the environmental balance and leading to contamination of the environment, including foods, with trace elements. The content of trace elements in foods depends on their concentration in the raw materials and additives used in food production. In addition, trace elements may be transmitted to food from the equipment used during food processing and from the packaging material during storage. Trace elements include Heavy Metals, some of which have recently received particular attention. Many definitions of ‘Heavy Metals’ have been put forward. The simplest and most precise describes Heavy Metals as all metal compounds of atomic weight over 20. Other definitions are based on the specific weight, and give the lower limits for Heavy Metals as 4.5, 5, or even 6 g per cm 3 . Due to toxicity of some Heavy Metals and the possibility of environmental contamina-tion, the potential for high risk is linked to Hg, Cd, As, Pb, as well as Cu, Zn, Sn, Cr, Ni. All elements are present in the environment (and also in plant and animal organisms, and in water and food) as salts or as metalo-organic compounds, and only in such forms are they biologically active. To limit the possibilities of food poisoning in humans caused by ingestion of excessive amounts of trace elements via food and water, highest allowable concentrations of trace elements are fixed. - Maria C. Hernandez-Soriano(Author)
- 2014(Publication Date)
- IntechOpen(Publisher)
The fate of the heavy metal in soils depends upon many soil processes that are governed by several soils properties of which soil pH and redox potential are known to be the most important parameters. Thus, the solubility of trace elements is often shown as a function of pH affected by amount and kind of organic matter. Trace elements are known to be accumu‐ lated in surface soils as a result of contamination from point sources as mining activities. An appreciable amount of the soils has been made unusable because of pollution. Highly conta‐ minated soils belong to a high healthy risk to human being and their environmentally harmful effects. That is why soil should be correctly understood and underestimated long range lethal effects that can have irreversible consequences. The improvement of soils damaged and contaminated by pollutants need of the particular soils, requires a full understanding of soil properties and of the deteriorating factors. Mercury is one of the most toxic elements to human health and ecosystem; because of all mercury species are toxic. A wide variety of mercury species exist in the environment and its © 2014 The Author(s). Licensee InTech. This chapter is distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. various chemical forms can differ in bioavailability, transport, persistence, and toxicity. Still, every mercury species is toxic with methyl mercury being the most toxic species. The World Health Organization (WHO) recommends a maximum methyl mercury intake of 1.6 µg Kg -1 per week, while the Environmental Protection Agency (EPA) lists a maximum recommended intake of 0.1 µg Kg -1 of body weight per day for adults. Due to high bioaccumulation, mercury is found on many levels of the food chain (Hinton and Veiga, 2001; Bengtsson, 2008).
Index pages curate the most relevant extracts from our library of academic textbooks. They’ve been created using an in-house natural language model (NLM), each adding context and meaning to key research topics.