
eBook - ePub
Chemical Analysis
Modern Instrumentation Methods and Techniques
Francis Rouessac, Annick Rouessac
This is a test
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Chemical Analysis
Modern Instrumentation Methods and Techniques
Francis Rouessac, Annick Rouessac
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Informazioni sul libro
Completely revised and updated, Chemical Analysis: Second Edition is an essential introduction to a wide range of analytical techniques and instruments. Assuming little in the way of prior knowledge, this text carefully guides the reader through the more widely used and important techniques, whilst avoiding excessive technical detail.
- Provides a thorough introduction to a wide range of the most important and widely used instrumental techniques
- Maintains a careful balance between depth and breadth of coverage
- Includes examples, problems and their solutions
- Includes coverage of latest developments including supercritical fluid chromatography and capillary electrophoresis
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Chemical Analysis è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Chemical Analysis di Francis Rouessac, Annick Rouessac in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Sciences physiques e Chimie analytique. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
PART 1
Separation methods
The invention of chromatography
Who invented chromatography, one of the most widely used laboratory techniques? This question leads to controversies. In the 1850s, Schönbein used filter paper to partially separate substances in solution. He found that not all solutions reach the same height when set to rise in filter paper. Goppelsröder (in Switzerland) found relations between the height to which a solution climbs in paper and its chemical composition. In 1861 he wrote ‘I am convinced that this method will prove to be very practical for the rapid determination of the nature of a mixture of dyes, especially if appropriately chosen and characterised reagents are used’.
Even if both of them did valuable work towards the progress of paper chromatography, it is traditional to assign the invention of modern chromatography to Michael S. Tswett, shortly after 1900. Through his successive publications, one can indeed reconstitute his thought processes, which makes of him a pioneer, even if not the inventor, of this significant separative method. His field of research was involved with the biochemistry of plants. At that time one could extract chlorophyll and other pigments from house plants, usually from the leaves, easily with ethanol. By evaporating this solvent, there remained a blackish extract which could be redissolved in many other solvents and in particular in petroleum ether (now one would say polar or non-polar solvents). However, it was not well understood why this last solvent was unable to directly extract chlorophyll from the leaves. Tswett put forth the assumption that in plants chlorophyll was retained by some molecular forces binding on the leaf substrate, thus preventing extraction by petroleum ether. He foresaw the principle of adsorption here. After drawing this conclusion, and to test this assumption he had the idea to dissolve the pigment extract in petroleum ether and to add filter paper (cellulose), as a substitute for leaf tissue. He realized that paper collected the colour and that by adding ethanol to the mixture one could re-extract these same pigments.
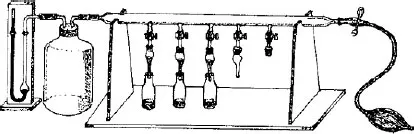
As a continuation of his work, he decided to carry out systematic tests with all kinds of powders (organic or inorganic), which he could spread out. To save time he had carried out an assembly which enabled him to do several assays simultaneously. He placed the packed powders to be tested in the narrow tubes and he added to each one of them a solution of the pigments in petroleum ether. That enabled him to observe that in certain tubes the powders produced superimposed rings of different colours, which testified that the force of retention varied with the nature of the pigments present. By rinsing the columns with a selection of suitable solvents he could collect some of these components separately. Modern chromatography had been born. A little later, in 1906, then he wrote the publication (appeared in Berichte des Deutschen Botanische Gesellshaft, 24, 384), in which he wrote the paragraph generally quoted: ‘Like light rays in the spectrum, the different components of a pigment mixture, obeying a law, are resolved on the calcium carbonate column and then can be measured qualitatively and quantitatively. I call such a preparation a chromatogram and the corresponding method the chromatographic method.’
1
General aspects of chromatography
Chromatography, the process by which the components of a mixture can be separated, has become one of the primary analytical methods for the identification and quantification of compounds in the gaseous or liquid state. The basic principle is based on the concentration equilibrium of the components of interest, between two immiscible phases. One is called the stationary phase, because it is immobilized within a column or fixed upon a support, while the second, called the mobile phase, is forced through the first. The phases are chosen such that components of the sample have differing solubilities in each phase. The differential migration of compounds lead to their separation. Of all the instrumental analytical techniques this hydrodynamic procedure is the one with the broadest application. Chromatography occupies a dominant position that all laboratories involved in molecular analysis can confirm.
1.1 General concepts of analytical chromatography
Chromatography is a physico-chemical method of separation of components within mixtures, liquid or gaseous, in the same vein as distillation, crystallization, or the fractionated extraction. The applications of this procedure are therefore numerous since many of heterogeneous mixtures, or those in solid form, can be dissolved by a suitable solvent (which becomes, of course, a supplementary component of the mixture).
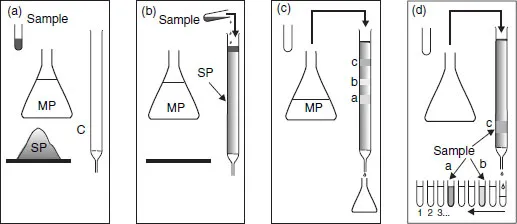
A basic chromatographic process may be described as follows (Figure 1.1):
1. A vertical hollow glass tube (the column) is filled with a suitable finely powdered solid, the stationary phase.
2. At the top of this column is placed a small volume of the sample mixture to be separated into individual components.
3. The sample is then taken up by continuous addition of the mobile phase, which goes through the column by gravity, carrying the various constituents of the mixture along with it. This process is called elution. If the components migrate at different velocities, they will become separated from each other and can be recovered, mixed with the mobile phase.
Figure 1.1 A basic experiment in chromatography. (a) The necessary ingredients (C, column; SP, stationary phase; MP, mobile phase; and S, sample); (b) introduction of the sample; (c) start of elution; (d) recovery of the products following separation.
This basic procedure, carried out in a column, has been used since its discovery on a large scale for the separation or purification of numerous compounds (preparative column chromatography), but it has also progressed into a stand-alone analytical technique, particularly once the idea of measuring the migration times of the different compounds as a mean to identify them had been conceived, without the need for their collection. To do that, an optical device was placed at the column exit, which indicated the variation of the composition of the eluting phase with time. This form of chromatography, whose goal is not simply to recover the components but to control their migration, first appeared around 1940 though its development since has been relatively slow.
The identification of a compound by chromatography is achieved by comparison: To identify a compound which may be A or B, a solution of this unknown is run on a column. Next, its retention time is compared with those for the two reference compounds A and B previously recorded using the same apparatus and the same experimental conditions. The choice between A and B for the unknown is done by comparison of the retention times.
In this experiment a true separation had not been effected (A and B were pure products) but only a comparison of their times of migration was performed. In such an experiment there are, however, three unfavourable points to note: the procedure is fairly slow; absolute identification is unattainable; and the physical contact between the sample and the stationary phase could modify its properties, therefore its retention times and finally the conclusion.
This method of separation, using two immiscible phases in contact with each other, was first undertaken at the beginning of the 20th century and is credited to botanist Michaël Tswett to whom is equally attributed the invention of the terms chromatography and chromatogram.
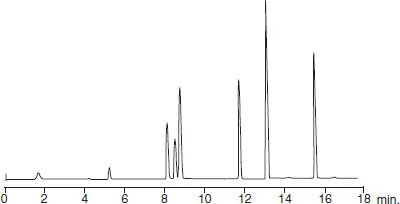
The technique has improved considerably since its beginnings. Nowadays chromatographic techniques are piloted by computer software, which operate highly efficient miniature columns able to separate nano-quantities of sample. These instruments comprise a complete range of accessories designed to assure reproducibility of successive experiments by the perfect control of the different parameters of separation. Thus it is possible to obtain, during successive analyses of the same sample conducted within a few hours, recordings that are reproducible to within a second (Figure 1.2).
The essential recording that is obtained for each separation is called a chromatogram. It corresponds to a two-dimensional diagram traced on a chart paper or a screen that reveals the variations of composition of the eluting mobile phase as it exits the column. To obtain this document, a sensor, of which there exists a great variety, needs to be placed at the outlet of the column. The detector signal appears as the ordinate of the chromatogram while time or alternatively elution volume appears on the abscissa.

Figure 1.2 The principle of analysis by chromatography. The chromatogram, the essential graph of every chromatographic analysis, describes the passage of components. It is obtained from variations, as a function of time, of an electrical signal emitted by the detector. It is often reconstructed from values that are digitized and store...
Indice dei contenuti
- Cover
- Contents
- Title Page
- Copyright
- Foreword to the first English edition
- Preface to the first English edition
- Preface to second edition
- Acknowledgments
- Introduction
- PART 1: Separation Methods
- PART 2: Spectroscopic Methods
- PART 3: Other Methods
- Solutions
- Appendix – List of acronyms
- Bibliography
- Table of some useful constants
- Index
Stili delle citazioni per Chemical Analysis
APA 6 Citation
Rouessac, F., & Rouessac, A. (2013). Chemical Analysis (2nd ed.). Wiley. Retrieved from https://www.perlego.com/book/1006908/chemical-analysis-modern-instrumentation-methods-and-techniques-pdf (Original work published 2013)
Chicago Citation
Rouessac, Francis, and Annick Rouessac. (2013) 2013. Chemical Analysis. 2nd ed. Wiley. https://www.perlego.com/book/1006908/chemical-analysis-modern-instrumentation-methods-and-techniques-pdf.
Harvard Citation
Rouessac, F. and Rouessac, A. (2013) Chemical Analysis. 2nd edn. Wiley. Available at: https://www.perlego.com/book/1006908/chemical-analysis-modern-instrumentation-methods-and-techniques-pdf (Accessed: 14 October 2022).
MLA 7 Citation
Rouessac, Francis, and Annick Rouessac. Chemical Analysis. 2nd ed. Wiley, 2013. Web. 14 Oct. 2022.