
eBook - ePub
Biotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation
Kenneth E. Avis,Vincent L. Wu
This is a test
- 404 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Biotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation
Kenneth E. Avis,Vincent L. Wu
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Informazioni sul libro
In this unique book, experts describe practices applicable to the large-scale processing of biotechnological products. Beginning with processing and bulk storage preservation techniques, the book provides strategies for improving efficiency of process campaigns of multiple products and manufacturing facilities for such processing techniques. Large-scale chromatography for the purification of biomolecules in manufacturing and lyophilization of protein pharmaceuticals are discussed. Includes a case study on blow-fill-seal processing technology and a chapter on economic and cost factors for bioprocess engineering.
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Biotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Biotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation di Kenneth E. Avis,Vincent L. Wu in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Business e Pharmaceutical, Biotechnology & Healthcare Industry. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
1
INTRODUCTION
Pharmaceutical products of biological origin, such as vaccines, have been known and utilized in therapy for human patients for many years. Only recently, products of biological origin with more definable composition and highly specific therapeutic effects have been developed. Normally, these products are administered to human patients by injection and must, therefore, meet all of the requirements for sterile parenteral products. Further, these products normally contain specific proteins and are identified as biotechnology products or, alternatively, as biopharmaceutical products. They are subject to the characteristic stability problems encountered with protein molecules. Therefore, purifying, compounding, processing, and preservation technologies essential for these biomolecules must be developed; these processes are quite distinctive from those for other sterile pharmaceutical products.
This book has been written to meet a need for practical information to aid researchers and scientists responsible for developing and implementing the unique technologies required for the large-scale preparation of sterile biotechnology products in accord with good manufacturing practices. Although not intended as an exhaustive treatise, this book emphasizes the pertinent technologies uniquely applicable to the large-scale preservation and processing of aqueous biopharmaceutical products. Because of product stability limitations, appropriate design and planning of process campaigning for multiple products is essential for efficient manufacturing operations. This book will identify unique ways in which efficient process campaigning is possible; it will also reveal aspects of appropriate facility design as well as certain economic considerations.
The design of process campaigning for aqueous biopharmaceutical products is dictated by their stability constraints. One of the most effective means of preserving biomolecules in aqueous systems for a limited, but often extended, period of time is freezing. However, freezing and subsequent thawing for further processing places significant stress on the protein molecules. Therefore, the bulk freezing and thawing of large volumes of in-process biopharmaceuticals must be designed and controlled very carefully. In chapter two of this book, Wisniewski and Wu discuss the requirements for this unique process. There are many advantages to storing an in-process protein product in a single, large container while awaiting further processing. However, significant factors that must be controlled in order to achieve a successful outcome of such processing include processing time, temperature and solute distribution in the solid and liquid phases, pH change, convectional and density stratification effects, and the possibility of recrystallization. Process considerations for achieving a successful outcome are the subjects of this chapter. The authors provide sufficient theoretical background information to substantiate the design requirements for the process. They also include considerations for the design and use of equipment, including details of the critical freezing and thawing steps to be performed. An extensive bibliography of 122 literature references is also provided.
An essential requirement for the safe and effective use of therapeutic proteins is very high product purity. In chapter three Wisniewski, Boschetti, and Jungbauer discuss process development, process design, and the design and operation concepts of the chromatographic method used to achieve the required high product purity on a large scale. Liquid chromatography has proven to be the optimal purification technique not only to achieve high product purity on a large scale but also to provide efficient productivity, process reliability, and robustness at a minimal cost. These criteria distinguish industrial chromatography from laboratory-scale preparative techniques where separation resolution is more prominent. The authors provide an exceptionally detailed and thorough description of chromatographic media, system hardware and other components, and system integration and performance. Sufficient theoretical concepts are presented to provide the background and substantiation for process development, scale-up, and optimization of the process. This very thorough chapter also contains an extensive bibliography (240 references) that reviews the subject matter.
The fourth chapter in this book is an exposé of the principles and the application of these principles to the preservation of protein molecules by the process of lyophilization (freeze-drying). Carpenter and Chang present a lucid and detailed account of the use of lyophilization as a means for removing water from a protein preparation in order to achieve long-term stability during packing, shipping, and storage prior to administration of the product to a human patient. However, the authors point out that without proper insight into the lyophilization process and how it affects proteins, it is not a simple task to remove water by freeze-drying without damaging the protein. Further, not only must the specific conditions for optimum protein stability be determined and established, but the appropriate nonspecific stabilizing additives required for the formulation must be determined. These subjects are thoroughly developed by the authors in this chapter. Organizationally, the authors first discuss the economical design of a lyophilization cycle that results in the desired cake properties and residual moisture. They next consider how to design formulations that stabilize proteins during both freezing and drying, including the mechanisms for stabilization by additives. The final section consists of a discussion of the optimization of formulations for long-term storage with a focus on the impact of physical properties of the dried solid on protein stability A literature review of 90 references concludes the chapter.
Achieving and maintaining the sterility and overall purity of parenteral products is a continuing focus of effort in good manufacturing practices by the pharmaceutical industry. One of the newer process methods utilized to improve the sterility assurance levels of aseptic processing is blow-fill-seal (b/f/s) technology. Essentially, the b/f/s process utilizes in-line machinery that forms a plastic container, fills it, and then seals it within a relatively small enclosure, with the critical environmental exposure operations protected with HEPA–filtered laminar flow air. In chapter five Wu and Leo present a case study in the qualification and use of a b/f/s system for the processing of a biopharmaceutical product. Issues reviewed include facility design, sterility assurance, validation, and the operational performance of the system. The authors point out that most of the issues explored are not limited to biotechnology products, but are applicable to pharmaceutical products in general. Specific concerns for the use of the system with sensitive protein products include assessment of heat imparted to the product, extractables from plastics, and product stability. A list of 7 literature references is provided.
In chapter six Hughes discusses an integrated approach for designing a pharmaceutical production facility for multiple products. The author states that the rationale for a multiproduct facility rather than one dedicated to a single product includes the following factors:
• The relatively small projected market for many new products
• The worldwide pressure to reduce costs for therapeutic agents
• New manufacturing technologies and equipment that have improved process control
• Advances in analytical techniques that have enhanced characterization of product in-process steps and the identification of contaminants and impurities
In this context the author aims to clarify the principle concerns of multiproduct processing, present the prevailing industry and regulatory viewpoints, suggest an integrated facility design development strategy, and review general biotechnology facility design requirements with an emphasis on the desirable features to include in a multiproduct facility. A list of 17 literature references is also given.
The final chapter, written by Wheelwright, provides an insight into economic and cost factors relative to engineering aspects of biotechnological processing. The author has divided the chapter into four main areas. The author begins by reviewing background factors and definitions. He then discusses the costs associated with development and its impact on later costs. This is followed by a description of facilities costs, including design and construction, and inherent considerations of operating costs. The author states that a vigilant evaluation of those factors that impact costs, especially future costs, at early stages of the development and planning process will have a significant impact on minimizing the cost as well as minimizing the time required to bring a new product to market. A comparison of fundamental questions, such as a dedicated plant versus a shared plant, new construction versus renovation, the number of purification steps versus process yield, and acceptable risk versus payback, allows quantification of economic return and maximization of value to investors. A list of 19 references concludes the chapter.
It is anticipated that the theoretical and practical information presented will make this book a highly useful tool for those involved in the large-scale processing and preservation of sterile biotechnological products. Since the topics covered are not exhaustive, additional topics will be covered in future books in the Drug Manufacturing Technology Series.
2
LARGE–SCALE FREEZING AND THAWING OF BIOPHARMACEUTICAL PRODUCTS
Protein products often have limited stability in the liquid state, yet may need to be stored for extended periods of time–for example, prior to lyophilization. Depending on the stability and storage period desired, biological products are often stored at 5°C, below −70°C, or as lyophilized product. The ability to freeze a bulk protein solution, and thereby hold it under stable conditions, offers significant economic and manufacturing advantages.
Freezing and thawing large volumes of bulk protein product has become an essential step in the manufacture of biopharmaceuticals at Genentech, Inc. The ability to freeze a product and hold it stable for extended periods of time provides flexibility for the manufacturing process and plays a key role in maximizing the productivity of multiproduct facilities for cell culture, purification, and filling and finishing. The process of freezing bulk product, followed by thawing at a later time, allows cell culture and purification activities to be conducted in a campaign mode: Production facilities focus on the manufacture of one product to build its inventory, while frozen bulk inventory of other products provides a steady supply of various products to the filling and finishing facilities. Figure 2.1 illustrates various steps in a typical biopharmaceutical manufacturing process where freezing hold steps may be implemented. Bulk material may be frozen in a concentrated form after final purification steps to minimize vessel storage requirements.
Figure 2.1. Bulk product may be frozen and stored after key process steps to provide manufacturing flexibility and to facilitate campaigning of multiproduct facilities. Savings in operating costs are realized when lots are pooled to produce larger and fewer final batches, thus reducing quality control expense and manufacturing labor.
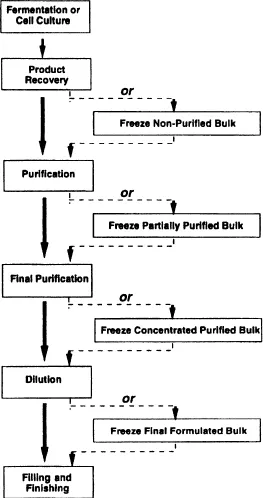
Once a frozen bulk inventory is established, an option exists of pooling multiple batches to produce larger lot sizes. There are significant savings realized by pooling product to produce larger and fewer batches of filled vials since the number of required quality control tests are reduced. Quality control costs for biotechnology products are a considerable part of the cost of goods. For example, quality control testing for the filled vial, not including quality control testing for the bulk product, is estimated to be in the range of 12–20 percent of the total cost of goods. However, the largest savings offered by the freezing and thawing process is realized due to the reduced capital investment in the construction of expensive facilities, since running the plant in a campaign mode and building a frozen bulk inventory may preclude the need for dedicated production facilities to produce a constant supply of bulk product. The ability to build a large inventory of frozen bulk product also makes it possible to provide adequate supply for the introductory market launch of a biotechnology product.
The use of a specially designed, stainless steel, portable freeze-thaw vessel, sized to the batch, was found to be suitable for the sterile storage and handling of bulk protein product at Genentech. Product transfer and filtration into a stainless steel portable vessel is consistent with current good pharmaceutical manufacturing operations. There are many advantages in storing product in a single, large container. Dividing the batch into multiple, smaller volumes may increase the possibility of product contamination, add operational steps, require additional quality control testing, and require careful batch tracking procedures to avoid product mixup from multiple batches. The stainless steel vessels lend themselves to clean-in-place (CIP) and steam-in-place (SIP) procedures, and provide a robust ...
Indice dei contenuti
- Cover
- Title Page
- Copyright Page
- Table of Contents
- Foreword
- 1. Introduction
- 2. Large-Scale Freezing and Thawing of Biopharmaceutical Products
- 3. Process Design Considerations for Large-Scale Chromatography of Biomolecules
- 4. Lyophilization of Protein Pharmaceuticals
- 5. Advances in Blow/Fill/Seal Technology: A Case Study in the Qualification of a Biopharmaceutical Product
- 6. Multiproduct Facility Design: An Integrated Approach
- 7. Economic and Cost Factors of Bioprocess Engineering
- Indexes
Stili delle citazioni per Biotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation
APA 6 Citation
[author missing]. (2020). Biotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation (1st ed.). CRC Press. Retrieved from https://www.perlego.com/book/1658855/biotechnology-and-biopharmaceutical-manufacturing-processing-and-preservation-pdf (Original work published 2020)
Chicago Citation
[author missing]. (2020) 2020. Biotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation. 1st ed. CRC Press. https://www.perlego.com/book/1658855/biotechnology-and-biopharmaceutical-manufacturing-processing-and-preservation-pdf.
Harvard Citation
[author missing] (2020) Biotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation. 1st edn. CRC Press. Available at: https://www.perlego.com/book/1658855/biotechnology-and-biopharmaceutical-manufacturing-processing-and-preservation-pdf (Accessed: 14 October 2022).
MLA 7 Citation
[author missing]. Biotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation. 1st ed. CRC Press, 2020. Web. 14 Oct. 2022.