Abstract:
The first recombinant protein therapeutic was Humulin®, recombinant human insulin, which was licensed in 1982. Today, there are four different types of biologics, including replacement protein therapeutics with enzymatic or regulatory activity, protein therapeutics with binding specific targets, protein-based prophylactic vaccines, and protein diagnostics. Monoclonal antibodies and Fc fusion proteins are the major forms of therapeutic proteins possessing binding activities. Monoclonal antibodies have two major functional domains, the FAb arms, which are responsible for binding the antigens, and the Fc domain, which interacts with the immune system. The potency of a monoclonal antibody depends on the strength of binding, the epitopes to which the antibodies bind, and the Fc activity. Monoclonal antibodies are named according to the sources from which they were obtained as well as the human component of the antibody.
1.1 Introduction
Emil von Behring, considered to be the father of immunology and winner of the first Nobel Prize in Medicine and Physiology in 1901, and Shibasaburo Kitasato, eventual founder of Japan’s famed Kitasato Institute, were the first to discover that a substance in blood was capable of neutralizing diphtheria toxin (Behring and Kitasato, 1890). This substance was subsequently named “Antikörper”, or “antibodies”, and was determined to have specificity for one toxin over another. In the following years, the terms “Antisomatogen” and “Immunkörperbildner” also were coined to describe the material that induced the formation of the Antikörper. The term “antigen” eventually arose from the combination of those names (Schroeder and Cavacini, 2010), and thus the foundation of immunology consisting of an antigen and its cognate antibody was laid for the next century of antibody research.
The field of genetically engineered therapeutic monoclonal antibodies (MAbs), the topic of this book, has been built on the shoulders of many inventions over decades of research, but two key discoveries in the mid-1970s stand out as seminal events that laid the groundwork for this field to exist as it does today. When Stanley Cohen, Herbert Boyer, and their colleagues made the first recombinant DNA molecules in 1973 by isolating a bacterial plasmid, cutting it site-specifically with the restriction endonuclease EcoRI, inserting foreign DNA into it, and then reforming the modified plasmid with DNA ligase (Cohen et al., 1973), they started a revolution that has changed the medical world in ways likely envisioned by only the wildest imaginations at that point in time. Virtually every new drug developed by the pharmaceutical industry today has been derived by a process that includes cloning and expression of the drug target, and in many cases, X-ray crystallographic analysis of the drug and recombinant target together. Likewise, the entire field of monoclonal antibodies derives from a key event, i.e. the discovery by Köhler and Milstein (1975) that murine B cells could be fused with murine myeloma cells to produce single fusion cell lines (hybridomas), which produce antibodies with a single, unique specificity (i.e. monoclonal antibodies). The field of genetically engineered MAbs, which marries standard molecular biology with antibody technologies, can be traced directly back to those two seminal papers.
Now, more than 35 years later, the field of genetically engineered MAbs, using technologies descended from both Cohen et al. (1973) and Köhler and Milstein (1975), is a rapidly maturing field in which hundreds of researchers in academia and in dozens of biotechnology and biopharmaceutical companies are engaged. Genetically engineered therapeutic antibodies have now been marketed for over a quarter century, starting with the United States Food and Drug Administration (FDA) approval of ReoPro® in December of 1984. Since that approval, an additional 39 recombinant therapeutic antibodies and related Fc fusion proteins (FcFPs) have been approved for marketing, with hundreds more now in clinical trials to follow them. This book describes the current status of therapeutic antibody engineering as well as the path to get to where we are today.
It also attempts to address the major issues, challenges, and opportunities we will face as we push forward into the second decade of the twenty-first century.
1.2 Definitions of biologies
Biologics have been defined by the FDA as “a virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product, or analogous product, or arsphenanaine, or derivative of arsphenamine (or any other trivalent organic arsenic compound), applicable to the prevention, treatment or cure of a disease or condition of human beings” (Public Health Services Act 42 USC § 262(i)). While this definition by statute is now antiquated, it does provide a broad guidance as to what may be included in the term “biologic.” Clearly, chemical compounds like arsphenines that were historically considered as biologics no longer fit the description. Leader et al. (2008) recently summarized the field of biologics, placing biopharmaceutical proteins into four major categories:
1. protein therapeutics with enzymatic or regulatory activity (e.g. replacement therapies such as insulin, growth hormone, Factor IX, β-glucocerebrosidase);
2. protein therapeutics with special targeting activity (e.g. MAbs or other binding proteins, including FcFPs, that bind specific therapeutic targets. Two examples are the anti-tumor necrosis factor (TNF)-α MAb, Remicade®, and the anti-TNF-α/β FcFP, Enbrel®);
3. protein-based prophylactic vaccines (e.g. human papilloma virus (HPV) vaccine made using virus-like particles containing HPV major capsid protein L1); and
4. protein diagnostics (e.g. biomarkers such as glucagon, and imaging agents such as technetium- or indium-conjugated antibodies) (Leader et al., 2008).
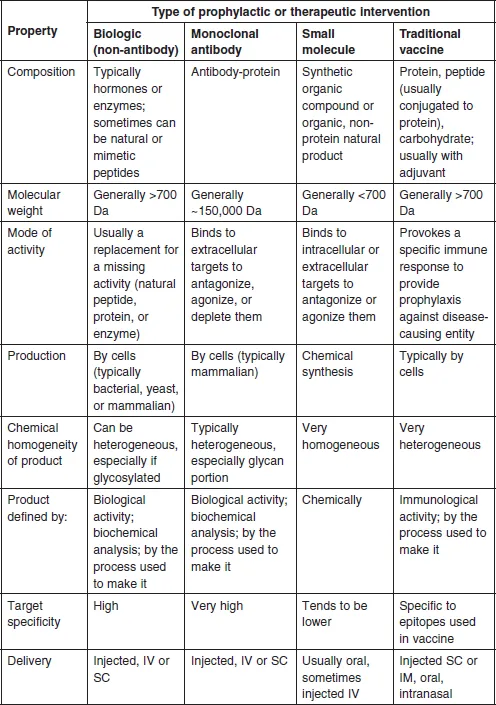
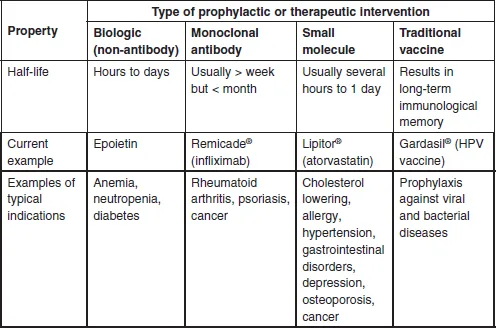
Figure 1.1 shows the fundamental structure of an immunoglobulin G (IgG), and a typical FcFP, etanercept, as compared with the non-antibody biologic, human growth hormone (HGH), the peptide hormone insulin, and the small molecule penicillin G. This book will focus largely on therapeutic MAbs and FcFPs, with some reference to the therapeutic protein history and markets as they relate to the development and acceptance of therapeutic MAbs by the industry. Table 1.1 defines some of the most significant similarities and differences between non-MAb biologics, MAbs, small molecules, and traditional vaccines. The key differences between biologics and small molecules lie in the types of targets addressed, molecular size and complexity, inherent toxicities, off-target activities, drug metabolism, pharmacokinetics, route of administration, method of manufacturing, and product homogeneity (Table 1.1). Biologics can be subdivided into three major categories: monoclonal antibody (MAb) products, non-MAb products, and vaccines. Since vaccines are used to stimulate an immune response and, at least historically, have been typically developed for prophylactic use rather than therapeutic use (Table 1.1), they will not be addressed further here.
Table 1.1
General properties of non-MAb biologics, MAbs, small molecules, and traditional vaccines
Abbreviations: Da: Dalto...