![]()
Chapter 1
Introduction
1.1 Global Energy Flow
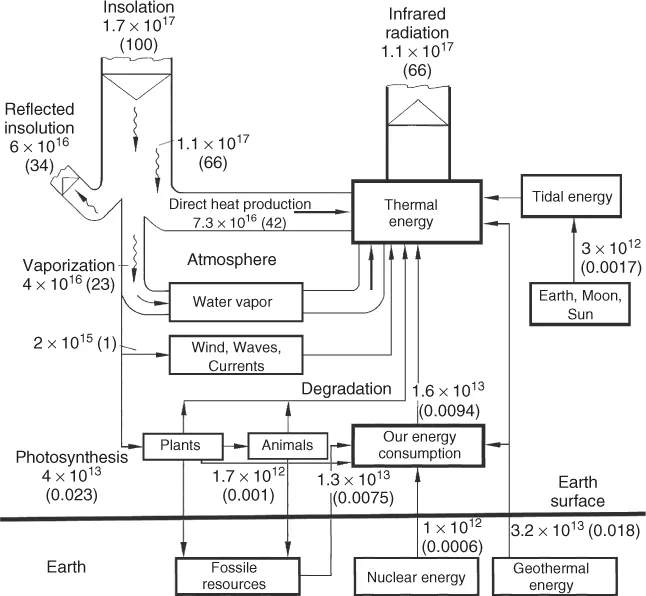
The global demand for primary energy has grown enormously during the past decades. It is now about 5.0 · 1020 J per year or 16 TW (Figure 1.1). Most of this energy is dissipated as waste heat. As the solar power reaching the Earth (insolation) is 170 000 TW, we recognize that, on a global scale, the heat dissipation caused by human activities is about 10 000 times smaller than the solar input. However, inside cities, the anthropogenic heat dissipation and the solar input can become comparable. This leads to a warmer microclimate.
1.2 Natural and Anthropogenic Greenhouse Effect
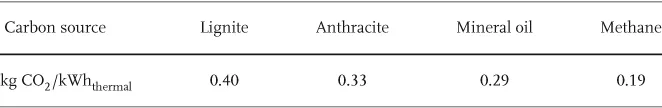
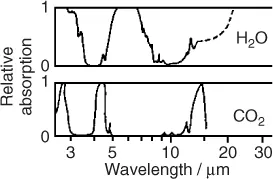
A much more severe and global problem associated with the flow of energy is the anthropogenic emission of greenhouse gases. Most important among these is carbon dioxide (CO2) released by burning of fossil carbon (Table 1.1). The average dwell time of CO2 in the atmosphere is about 120 years. CO2 is a natural constituent of the atmosphere together with water vapor, the latter being the dominant greenhouse gas. These gases interact with a thermal radiation of 1.1 · 1017 W or about 220 W/m2 from the Earth (Figures 1.1 and 1.2). Their molecules either have a permanent electric dipole moment, as with H2O, or are vibrationally excited, as in the case of CO2 and CH4, another greenhouse gas.
Table 1.1 The amount of CO2 emitted per thermal kilowatt hour depends strongly on the atomic carbon/hydrogen ratio of the fossil fuel (1 kg of C is oxidized into 3.7 kg CO2).
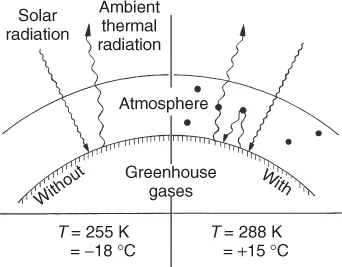
These gases thus reduce the radiative heat transfer from the Earth into space, raising the global mean temperature from −18 to +15 °C, a precondition for a habitable Earth. A stable mean temperature requires a balance between solar input and thermal output (Figure 1.3).
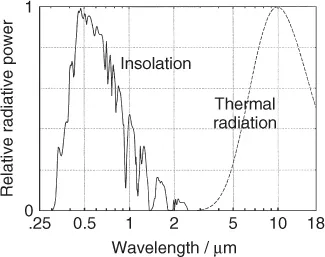
It is important to answer the question why the concentration of CO2 is of any consequence. After all, the concentration of water vapor is about 100 times larger. Figure 1.4 shows that some of the absorption bands of CO2 coincide with “windows” in the H2O spectrum. Thus, a relatively small amount of CO2 can reduce the thermal flow, that would otherwise escape into space through these windows. The effect of the other greenhouse gases on the thermal flow into space is characterized by the global warming potential (GWP). For example, CH4 has a GWP ≈ 25, indicating that one molecule of CH4 is 25 times more effective than one molecule of CO2.
CO2 and other noncondensing greenhouse gases together account for about 25% of the terrestrial greenhouse effect. Atmospheric modeling [3] shows that these gases via feedback processes provide the necessary infrared absorption to sustain the present levels of water vapor and clouds, which make up the remaining 75% of the terrestrial greenhouse effect. (Without CO2 and the other noncondensing greenhouse gases, the atmospheric water vapor would condense. The terrestrial greenhouse would collapse within a few decades, sending Earth into an ice-bound state.)
In summary, the natural greenhouse effect determined the climate on the Earth in the past and supported the development of life. About 150 years of anthropogenic activities, however, accompanied by the burning of coal, oil, and natural gas, have led to a drastic increase in the concentration of greenhouse gases in the atmosphere. This is causing an additional, human-related reduction in the thermal radiation transfer to space. The imbalance, also called radiative forcing, is about 1 W/m2 today [4]. This is only a 0.5% contribution to the total radiative heat transfer from the Earth. Furthermore, the large thermal mass of the oceans has stored large amounts of heat. Nonetheless, a global warming of about 0.8 K since 1870 and 0.6 K since about 1960 is observed.
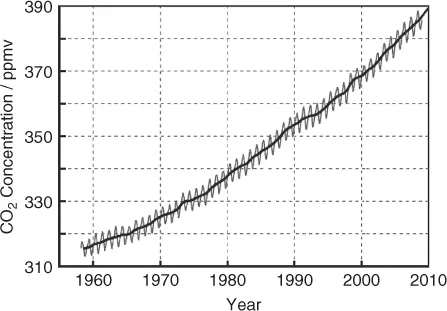
The main culprit for the warming of the Earth is anthropogenic CO2. Its concentration in the atmosphere rose from a preindustrial value of 280 to about 390 ppm in the year 2010 (Figure 1.5).
In order to put the anthropogenic influence on our climate in perspective, we have to look at the history of CO2. The CO2 concentration during the past 400 000 years fluctuated between about 180 and 280 ppm, and never exceeded 300 ppm. A higher CO2 concentration was always accompanied by warmer temperatures and vice versa. The increase to 390 ppm thus is rather dramatic. The retreat of mountain glaciers and the north polar ice sheet appear to be manifestations of the problem.
Problem 1.1
A warmer atmosphere can hold more moisture [5] and thus more torrential rains can be expected. Calculate the relative increase in water vapor pressure for an atmosphere at 20 °C, assuming a temperature increase of 1 K. The exponential dependence of vapor pressure on temperature is p(T) = p0 · exp[−ΔE/(kB · T)]. (In order to find ΔE, start with the mass specific heat of vaporization, then find the molar mass of water and the number of water molecules per mole.)
We should mention here that aerosols in the atmosphere are responsible for a negative radiative forcing. Combustion caused by humans has increased the amount of atmospheric aerosols substantially. The interaction between these aerosols and solar radiation leads to a direct cooling of the atmosphere. In addition, aerosols enhance the condensation of moisture and modify the optical properties of clouds. The sign of this indirect aerosol effect – whether positive or negative – is still uncertain. A third indirect aerosol effect involves the change of biochemical cycles [6]. All three effects may have reduced global warming substantially. Anticipating future worldwide installations of scrubbing devices, much higher CO2 mitigation costs could result than previously thought.
1.3 Limit to Atmospheric CO2 Concentration
In order to prevent catastrophic climate changes in the future, causing, for example, a rise in sea level of several meters, the CO2 concentration in the atmosphere will have to be limited. The actual limit is the subject of much discussion at present.
As an example, let us consider a maximum tolerable CO2 concentration of 560 ppm, that is, twice the preindustrial value. From the known global annual us...