
Introduction to Physical Polymer Science
Leslie H. Sperling
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Introduction to Physical Polymer Science
Leslie H. Sperling
About This Book
An Updated Edition of the Classic Text
Polymers constitute the basis for the plastics, rubber, adhesives, fiber, and coating industries. The Fourth Edition of Introduction to Physical Polymer Science acknowledges the industrial success of polymers and the advancements made in the field while continuing to deliver the comprehensive introduction to polymer science that made its predecessors classic texts.
The Fourth Edition continues its coverage of amorphous and crystalline materials, glass transitions, rubber elasticity, and mechanical behavior, and offers updated discussions of polymer blends, composites, and interfaces, as well as such basics as molecular weight determination. Thus, interrelationships among molecular structure, morphology, and mechanical behavior of polymers continue to provide much of the value of the book.
Newly introduced topics include:
- Nanocomposites, including carbon nanotubes and exfoliated montmorillonite clays
- The structure, motions, and functions of DNA and proteins, as well as the interfaces of polymeric biomaterials with living organisms
- The glass transition behavior of nano-thin plastic films
In addition, new sections have been included on fire retardancy, friction and wear, optical tweezers, and more.
Introduction to Physical Polymer Science, Fourth Edition provides both an essential introduction to the field as well as an entry point to the latest research and developments in polymer science and engineering, making it an indispensable text for chemistry, chemical engineering, materials science and engineering, and polymer science and engineering students and professionals.
Frequently asked questions
Information
CHAPTER 1
INTRODUCTION TO POLYMER SCIENCE
1.1 FROM LITTLE MOLECULES TO BIG MOLECULES
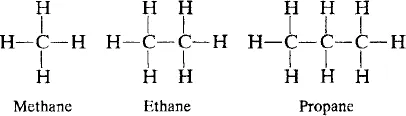
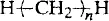
| Number of Carbons in Chain | State and Properties of Material | Applications |
| 1–4 | Simple gas | Bottled gas for cooking |
| 5–11 | Simple liquid | Gasoline |
| 9–16 | Medium-viscosity liquid | Kerosene |
| 16–25 | High-viscosity liquid | Oil and grease |
| 25–50 | Crystalline solid | Paraffin wax candles |
| 50–1000 | Semicrystalline solid | Milk carton adhesives and coatings |
| 1000–5000 | Tough plastic solid | Polyethylene bottles and containers |
| 3−6 × 105 | Fibers | Surgical gloves, bullet-proof vests |