
eBook - ePub
Fundamentals of Materials Engineering - A Basic Guide
Shashanka Rajendrachari, Orhan Uzun
This is a test
Share book
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Fundamentals of Materials Engineering - A Basic Guide
Shashanka Rajendrachari, Orhan Uzun
Book details
Book preview
Table of contents
Citations
About This Book
Fundamentals of Materials Engineering - A Basic Guide is a helpful textbook for readers learning the basics of materials science. This book covers important topics and fundamental concepts of materials engineering including crystal structure, imperfections, mechanical properties of materials, polymers, powder metallurgy, corrosion and composites. The authors have explained the concepts in an effective way and by using simple language for the benefit of a broad range of readers. This book is also beneficial to the students in engineering courses at B.Sc, M.Sc, and M.Tech. levels.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Fundamentals of Materials Engineering - A Basic Guide an online PDF/ePUB?
Yes, you can access Fundamentals of Materials Engineering - A Basic Guide by Shashanka Rajendrachari, Orhan Uzun in PDF and/or ePUB format, as well as other popular books in Technik & Maschinenbau & Werkstoffwissenschaft. We have over one million books available in our catalogue for you to explore.
Information
Subtopic
WerkstoffwissenschaftThe Structure Of Materials
R. Shashanka, Orhan Uzun
Abstract
Do you know all the materials are made up of atoms? And there are different types of atoms named as elements? Have you ever wondered why an atom consists of electrons, protons, and neutrons? Do you know the number of electrons in the outer shell depicts the number of atoms present? Can you tell me why metals are ductile compared to hard and brittle ceramics? Why ductile iron becomes harder when we add a small amount of carbon? Why some materials act as conductors of heat and electric current and some are insulators [1]? To understand all these concepts one should learn the atomic structure, chemical bonding, structure of amorphous and crystalline materials. The present chapter describes all these concepts in a very simple way.
Keywords: Amorphous materials, BCC, Covalent bonding, Crystalline materials, FCC, Ionic bonding, Lattice points, Macrostructure, Metallic bonding, Micro-structure, Nanostructure, Radius ratio, Unit cells, Van der Waals bonding.
1. INTRODUCTION
In this chapter, we will discuss how the microstructure, composition, and processing method of the materials control their different properties. The structure of an atom is shown in Fig. (1) [2].
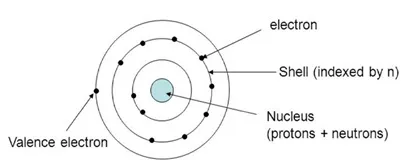
Structure of an atom [2].
The structures of materials are classified mainly based upon their sizes; (i) atomic structure, (ii) microstructure, and (iii) macrostructure as shown in Fig. (2) [2-4].
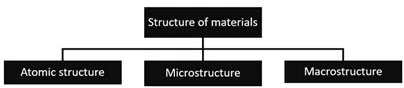
Classification of materials.
The atomic structure consists of those features that cannot be seen through our naked eyes; like bonding between atoms and how the atoms are arranged.
As we know, the atoms are composed of electrons, protons, and neutrons, where, electrons are negatively charged particles and protons are positively charged particles, neutrons have a neutral charge. The magnitude of each charged particle in an atom is 1.6×10-19 Coulombs. Both neutrons and protons have almost the same masses; whereas electrons show negligible mass. The unit of mass is an atomic mass unit (amu) =1.66×10-27 kg and equals 1/12th mass of a carbon atom. The Carbon nucleus has Z=6, and A=6, where 'Z' is the number of protons, and 'A' the number of neutrons. Neutrons and protons have very similar masses, roughly equal to 1 amu each [5-7]. A neutral atom has the same number of electrons and protons, Z.
Microstructure involves the features that can be easily seen by using an optical or electron microscope as shown in Fig. (3) [8]. The word “microstructure” is used to explain the appearance of the materials on the nanometer-centimeter length scale. When we describe the microstructure of the material; it is very important to consider the length scale because different length scales will give different microstructural features [8].
Macrostructure is the one that can be seen with little or no magnification or can be seen with naked eyes. Generally, the macrostructure investigations are performed on flat samples, called templates, and also on the fracture surfaces of the materials. To investigate the topography of the structure, the surface of the template is polished and carefully etched with acid or alkaline solutions. The macrostructure study reveals the discontinuity of the metal surface due to cavities, gas bubbles, porosity, etc.; and it also reveals the distribution of impurities, crystallites, and inclusions in different parts of the material. The macrostructure study plays an important role in determining the initial quality of the materials [9].
The chemical, physical, thermal, electrical, magnetic, and optical properties of all materials mainly depend upon their structure. Nevertheless, micro and macrostructure not only influence mechanical but also other properties. Atomic bonding affects the strength of metals by holding the atoms together by strong bonds. As we know that metals can be formable only when bonds allow atoms to move freely inside the materials. To understand this, we should discuss how the atoms are arranged and bonded in a material.

Microstructure of steel [8].
2. ATOMIC BONDING
To understand the behavior of materials and wide differences in their properties, it is neces...