![]()
1
Introduction
Chemical process technology has had a long, branched road of development. Processes such as distillation, dyeing, and the manufacture of soap, wine, and glass have long been practiced in small-scale units. The development of these processes was based on chance discoveries and empiricism rather than thorough guidelines, theory, and chemical engineering principles. Therefore, it is not surprising that improvements were very slow. This situation persisted until the seventeenth and eighteenth centuries.1 Only then were mystical interpretations replaced by scientific theories.
It was not until the 1910s and 1920s, when continuous processes became more common, that disciplines such as thermodynamics, material and energy balances, heat transfer, and fluid dynamics, as well as chemical kinetics and catalysis became (and still are) the foundations on which process technology rests. Allied with these are the unit operations including distillation, extraction, and so on. In chemical process technology various disciplines are integrated. These can be divided according to their scale (Table 1.1).
Table 1.1 Chemical process technology disciplines
| Scale independent | Chemistry, biology, physics, mathematics |
| Thermodynamics |
| Physical transport phenomena |
| Micro/nanolevel | Kinetics |
| Catalysis on a molecular level |
| Interface chemistry |
| Microbiology |
| Particle technology |
| Mesolevel | Reactor technology |
| Unit operations |
| Macrolevel | Process technology and process development |
| Process integration and design |
| Process control and operation |
Of course, this scheme is not complete. Other disciplines, such as applied materials science, information science, process control, and cost engineering, also play a role. In addition, safety is such an important aspect that it may evolve as a separate discipline.
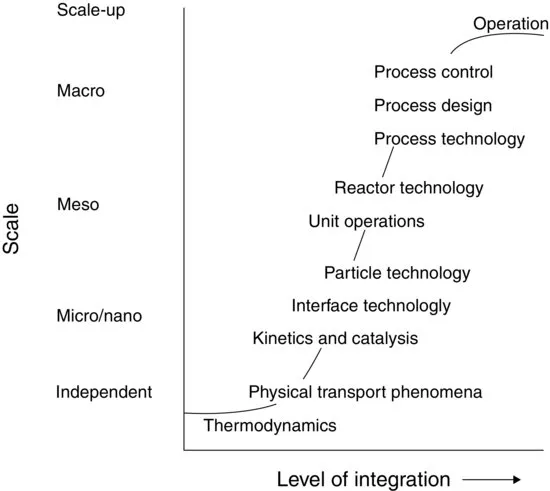
In the development stage of a process or product all necessary disciplines are integrated. The role and position of the various disciplines perhaps can be better understood from Figure 1.1, in which they are arranged according to their level of integration. In process development, in principle the x-axis also roughly represents the time progress in the development of a process. The initial phase depends on thermodynamics and other scale-independent principles. As time passes, other disciplines become important, for example, kinetics and catalysis on a micro/nanolevel, reactor technology, unit operations and scale-up on the mesolevel, and process technology, process control, and so on on the macrolevel.
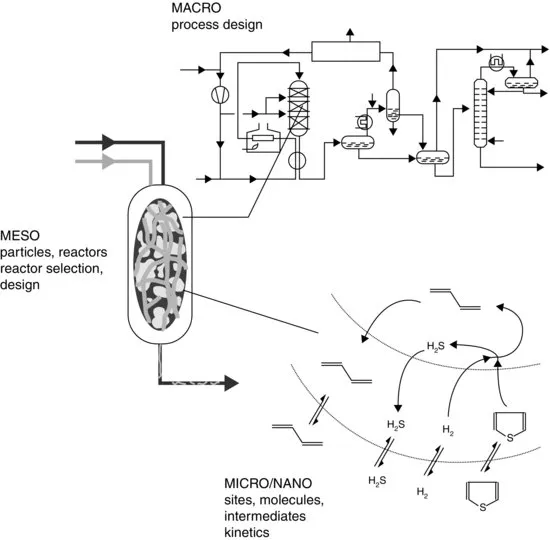
Of course, there should be intense interaction between the various disciplines. To be able to quickly implement new insights and results, these disciplines are preferably applied more or less in parallel rather than in series, as can also be seen from Figure 1.1. Figure 1.2 represents the relationship between the different levels of development in another way. The plant is the macrolevel. When focusing on the chemical conversion, the reactor would be the level of interest. When the interest goes down to the molecules converted, the micro-and nanolevels are reached.
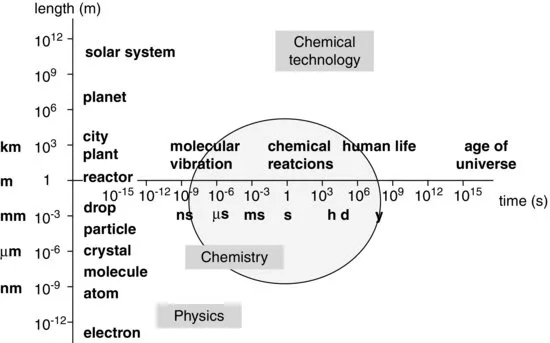
An enlightening way of placing the discipline Chemical Engineering in a broader framework has been put forward by Villermeaux [personal communication], who made a plot of length and time scales (Figure 1.3). From this figure it can be appreciated that chemical engineering is a broad integrating discipline. On the one hand, molecules, having dimensions in the nanometer range and a vibration time on the nanosecond scale, are considered. On the other hand, chemical plants may have a size of half a kilometer, while the life expectancy of a new plant is 10–20 years. Every division runs the danger of oversimplification. For instance, the atmosphere of our planet could be envisaged as a chemical reactor and chemical engineers can contribute to predictions about temperature changes and so on by modeling studies analogous to those concerning “normal” chemical reactors. The dimensions and the life expectancy of our planet are fortunately orders of magnitude larger than those of industrial plants.
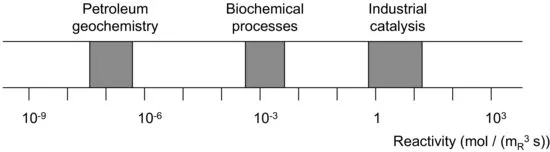
Rates of chemical reactions vary over several orders of magnitude. Processes in oil reservoirs might take place on a time scale of a million years, processes in nature are often slow (but not always), and reactions in the Chemical Process Industry usually proceed at a rate that reactor sizes are reasonable, say smaller than 100 m3. Figure 1.4 indicates the very different productivity of three important classes of processes.
It might seem surprising that despite the very large number of commercially attractive catalytic reactions, the commonly encountered reactivity is within a rather narrow range; reaction rates that are relevant in practice are rarely less than one and seldom more than ten mol mR3 s−1 for oil refinery processes and processes in the chemical industry. The lower limit is set by economic expectations: the reaction should take place in a reasonable amount of (space) time and in a reasonably sized reactor. What is reasonable is determined by physical (space) and economic constraints. At first sight it might be thought that rates exceeding the upper limit are something to be happy about. The rates of heat and mass transport become limiting, however, when the intrinsic reaction rate far exceeds the upper limit.
A relatively recent concept is that of Process Intensification, which aims at a drastic decrease of the sizes of chemical plants [2,3]. Not surprisingly, the first step often is the development of better catalysts, that is, catalysts exhibiting higher activity (reactor volume is reduced) and higher selectivity (separation section reduced in size). As a result, mass and heat transfer might become rate determining and equipment allowing higher heat and mass transfer rates is needed. For instance, a lot of attention is given to the development of compact heat exchangers that allow high heat transfer rates on a volume basis. Novel reactors are also promising in this respect, for instance monolithic reactors and microreactors. A good example of the former is the multiphase monolithic reactor, which allows unusually high rates ...