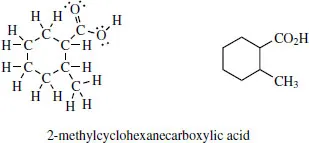![]()
1
Molecular representations and nomenclature
1.1 Molecular representations
From the beginnings of chemistry, scientists have devised means of representing the materials they are discussing, and have gradually developed a comprehensive range of shorthand notations. These cover the elements themselves, bonding between atoms, the arrangement of atoms in molecules, and, of course, a systematic way of naming compounds that is accepted and understood throughout the scientific world.
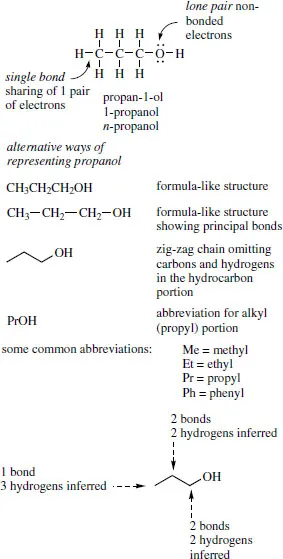
The study of carbon compounds provides us with the subdivision ‘organic chemistry’, and a few simple organic compounds can exemplify this shorthand approach to molecular representations. The primary alcohol propanol (systematically propan-1-ol or 1-propanol, formerly n-propanol, n signifying normal or unbranched) can be represented by a structure showing all atoms, bonds, and lone pair or nonbonding electrons.
Lines are used to show what we call single bonds, indicating the sharing of one pair of electrons. In writing structures, we have to remember the number of bonds that can be made to a particular atom, i.e. the valency of the atom. In most structures, carbon is tetravalent, nitrogen trivalent, oxygen divalent, and hydrogen and halogens are univalent. These valencies arise from the number of electrons available for bonding. More often, we trim this type of representation to one that shows the layout of the carbon skeleton with attached hydrogens or other atoms. This can be a formula-like structure without bonds, or it can be one showing just the principal bonds, those of the carbon chain.
However, for many complex structures, even these approaches become too tedious, and we usually resort to a shorthand version that omits most, if not all, of the carbon and hydrogen atoms. Propanol is now shown as a zig-zag chain with an OH group at one end. The other end of the chain, where it stops, is understood to represent a methyl group; three attached hydrogens have to be inferred. At a point on the chain, two hydrogens are assumed, because two bonds to carbons are already shown. In a structure where three bonds joined, a single additional hydrogen would be assumed (see vinyl chloride, below).
The zig-zag arrangement is convenient so that we see where carbons are located (a long straight line would not tell us how many carbons there are), but it also mimics the low-energy arrangement (conformation) for such a compound (see Section 3.3.1). Note that it is usual to write out the hydroxyl, or some alternative group, in full. This group, the so-called functional group, tends to be the reactive part of the molecule that we shall be considering in reactions. When we want an even more concise method of writing the molecule, abbreviations for an alkyl (or aryl) group may be used, in which case propanol becomes PrOH. Some more common abbreviations are given later in Table 1.3.
Double bonds, representing the sharing of two pairs of electrons, are inferred by writing a double line. Vinyl chloride (systematically chloroethene) is shown as two different representations according to the conventions we have just seen for propanol. Note that it is customary always to show the reactive double bond, so that CH2CHCl would not be encountered as an abbreviation for vinyl chloride.
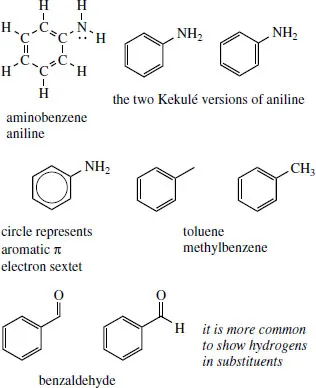
The six-membered cyclic system in aromatic rings is usually drawn with alternating double and single bonds, i.e. the Kekulé form, and it is usually immaterial which of the two possible versions is used. Aniline (systematically aminobenzene or benzenamine) is shown with and without carbons and hydrogens. It is quite rare to put in any of the ring hydrogens on an aromatic ring, though it is sometimes convenient to put some in on the substituent, e.g. on a methyl, as in toluene (methylbenzene), or an aldehyde group, as in benzaldehyde.
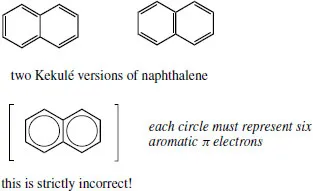
Benzene strictly does not have alternating double and single bonds, but the aromatic sextet of electrons is localized in a π orbital system and bond lengths are somewhere in between double and single bonds (see Section 2.9.4). To represent this, a circle may be drawn within the hexagon. Unfortunately, this version of benzene becomes quite useless when we start to draw reaction mechanisms, and most people continue to draw benzene rings in the Kekulé form. In some cases, such as fused rings, it is actually incorrect to show the circles.
Thus, naphthalene has only 10 π electrons, one from each carbon, whereas the incorrect two-circle version suggests it has 12 π electrons.
We find that, in the early stages, students are usually happier to put in all the atoms when drawing structures, following earlier practices. However, you are urged to adopt the shorthand representations as soon as possible. This saves time and cleans up the structures of larger molecules. Even a relatively simple molecule such as 2-methylcyclohexanecarboxylic acid, a cyclohexane ring carrying two substituents, looks a mess when all the atoms are put in. By contrast, the line drawing looks neat and tidy, and takes much less time to draw.
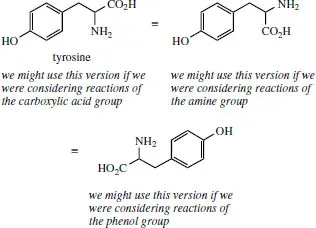
Do appreciate that there is no strict convention for how you orientate the structure on paper. In fact, we will turn structures around, as appropriate, to suit our needs. For example, the amino acid tyrosine has three functional groups, i.e. a carboxylic acid, a primary amine, and a phenol. How we draw tyrosine will depend upon what modifications we might be considering, and which functional group is being altered.
You will need to be able to reorientate structures without making mistakes, and also to be able to recognize different versions of the same thing. A simple example is with esters, where students have learnt that ethyl acetate (ethyl ethanoate) can be abbreviated to CH3CO2C2H5. When written backwards, i.e. C2H5OCOCH3, the ester functionality often seems less recognizable.
1.2 Partial structures
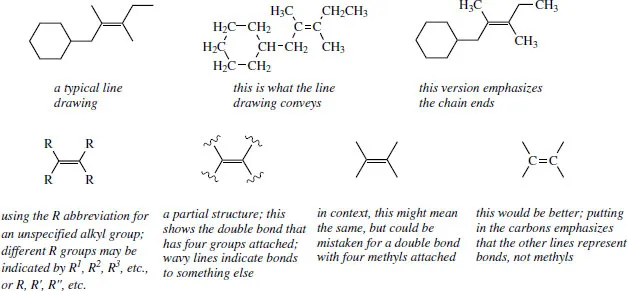
We have just seen that we can save a lot of time and effort by drawing structures without showing all of the atoms. When we come to draw reaction sequences, we shall find that we are having to repeat large chunks of the structure each time, even though no chemical changes are occurring in that part of the molecule. This is unproductive, so we often end up writing down just that part of the structure that is of interest, i.e. a partial structure. This will not cause problems when you do it, but it might when you see one and wish to interpret it.
In the representations overleaf, you can see the line drawing and the version with methyls that stresses the bond ends. Both are satisfactory. When we wish to consider the reactivity of the double bond, and perhaps want to show that reaction occurs irrespective of the alkyl groups attached to the double bond, we put in the abbreviation R (see below), or usually just omit them. When we omit the attached groups, it helps to show what we mean by using wavy lines across the bonds, but in our urge to proceed we tend to omit even these indicators. This may cause confusion in that we now have what looks like a double bond with four methyls attached, not at all what we intended. A convenient ploy is to differentiate this from a line drawing by putting in the alkene carbons.
1.3 Functional groups
The reactivity of a molecule derives from its functional group or groups. In most instances the hydrocarbon part of the molecule is likely to be unreactive, and the reactivity of the functional group is largely independent of the nature of the hydrocarbon part. In general terms, then, we can regard a molecule as R–Y or Ar–Y, a combination of a functional group Y with an alkyl group R or aryl group Ar that is not participating in the reaction under consideration. This allows us to discuss reactivity in terms of functional groups, rather than the reactivity of individual compounds. Of course, most...