
eBook - ePub
Essential Fluid, Electrolyte and pH Homeostasis
Gillian Cockerill, Stephen Reed
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Essential Fluid, Electrolyte and pH Homeostasis
Gillian Cockerill, Stephen Reed
Book details
Book preview
Table of contents
Citations
About This Book
This textbook provides a unique, pocket-sized, self-directed study guide to fluid, electrolyte and acid base homeostasis for undergraduate biomedical science, pharmacology, medical and allied health students. It details the chemical (mostly ionic) composition of body fluids, explains how abnormalities arise, what laboratory tests can be used to identify and analyze the cause of these disorders and shows how normality can be achieved to maintain health.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Essential Fluid, Electrolyte and pH Homeostasis an online PDF/ePUB?
Yes, you can access Essential Fluid, Electrolyte and pH Homeostasis by Gillian Cockerill, Stephen Reed in PDF and/or ePUB format, as well as other popular books in Scienze biologiche & Biotecnologia. We have over one million books available in our catalogue for you to explore.
Information
Part 1
Background theory and basic concepts
Overview
The purpose of Part 1 is to review some important concepts of physical chemistry and to introduce key ideas of physiology, all of which will provide underpinning knowledge for deeper study in Parts 2 and 3. Although some understanding of solutions, acids, bases, pH and buffers may have been acquired from previous studies, these topics are included here for revision; some readers may choose to omit certain sections.
An overview is given of body fluid compartments, their volumes and their chemical compositions. Importantly, concepts relating to osmotic balance and electrical neutrality of physiological fluids are also discussed.
SECTION 1.i
Introduction and overview
The human body is, by weight, predominantly water: the total volume1 being distributed into two major compartments. The larger proportion is located inside cells (intracellular fluid, ICF) with a smaller volume occurring as extracellular fluid (ECF). To function effectively, cells must maintain correct fluid volume balance, ionic balance, osmotic balance and acid-base balance. Two fundamental physicochemical phenomena, namely electroneutrality and osmosis (‘osmoneutrality’), have significant effects on cellular function. Homeostatic mechanisms operate to maintain physiological steady-state conditions of ionic and solute concentrations.
Body fluids are complex ‘cocktails’ of various chemicals such as (a) ions (electrolytes), notably sodium, potassium, calcium, chloride, phosphate and bicarbonate, (b) small molecular weight metabolites such as glucose, urea, urate (uric acid) and creatinine, and (c) larger molecular weight compounds, for example, proteins and lipoprotein complexes.
Qualitatively, the chemical composition of most body fluids is substantially the same, but quantitatively, the chemical content of the different body fluids varies considerably. Overall, the total volume of water in our bodies does not vary greatly, and nor does the overall chemical composition of the fluids, as both volume and composition are carefully regulated to maintain homeostasis. However, as is often the case in physiological systems, there is at the molecular level a dynamic state of flux and continual change occurring, with fluid and solutes moving between compartments. These movements are driven by physicochemical gradients which arise due to osmotic, electrochemical and concentration2 differences across cell membranes. Fluid movement between the intracellular and extracellular compartments is directed by osmosis (a particular form of passive diffusion), but because the outer plasma membrane of cells is relatively impermeable to most solutes, especially ions, active or passive carrier mechanisms are required to transport such components between compartments.
Figure 1.1 Membrane transport. Passive osmotic, passive mediated and active mediated mechanisms
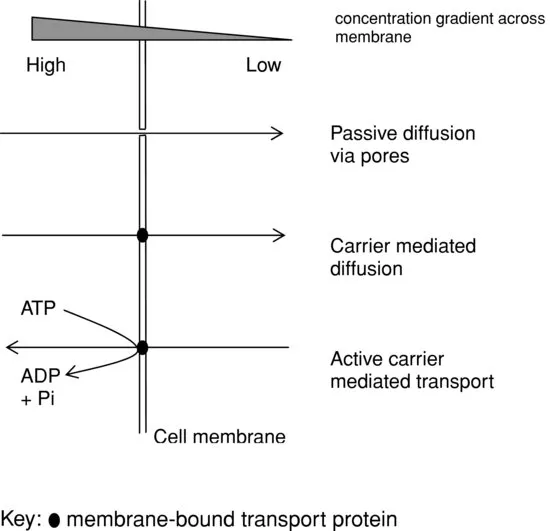
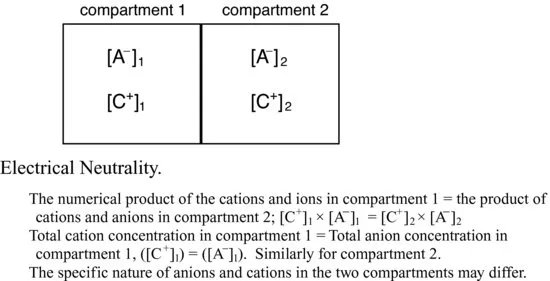
Electrochemical gradients across cell membranes arise due to the number and nature (principally their size and charge) of the solutes distributed on either side of the membrane. An imbalance in electrical charge across certain membranes is physiologically essential for example to allow nerve impulse conduction and for the initiation of muscle contraction, for example. However, for most cells, an equal distribution of total number of anions and cations is the norm. In addition to the necessity for electrical neutrality across a membrane, i.e. between compartments, the numbers of negative and positive charges within a particular compartment or body fluid must also be equivalent.
Normal hydrogen ion concentration in most body fluids is very low, in the nanomolar range, compared with concentrations of other ions such as sodium and potassium which are present at millimolar concentrations. Homeostatic mechanisms that regulate hydrogen ion balance are of necessity very sensitive to avoid the wide fluctuations in pH which might seriously impair enzyme function, leading to consequent cell dysfunction.
Figure 1.2 Ionic balance within and between compartments
Physiological control of body fluid volumes and their chemical composition is fundamental to the health and wellbeing of cells, tissues and whole organisms, and as such represents a major purpose of homeostasis. Several physiological systems are involved with normal fluid and electrolyte homeostasis, and thus disorders of the kidneys, liver, endocrine system and gut can all lead to fluid, electrolyte or pH imbalance.
The organs that play the most significant role in fluid and electrolyte homeostasis are the kidneys, which process approximately 140 litres of fluid containing a significant quantity of solutes, including electrol...