
eBook - ePub
Steroid Biochemistry
Volume II
R. Hobkirk
This is a test
Share book
- 206 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Steroid Biochemistry
Volume II
R. Hobkirk
Book details
Book preview
Table of contents
Citations
About This Book
An attempt has been made to attract contributions which illustrate the importance of certain enzymatic processes involved in steroid biosynthesis and metabolism and, in some cases, leading to steroidal action in target sites. Investigators actively engaged in research in such areas were invited to present their material in a manner which they considered fitting. It is hoped that as a result if this, the publication will possess sufficient depth to warrant approval. The blend of review material and experimental data originating in the author laboratories will, it is felt, make for useful reading.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Steroid Biochemistry an online PDF/ePUB?
Yes, you can access Steroid Biochemistry by R. Hobkirk in PDF and/or ePUB format, as well as other popular books in Medicine & Alternative & Complementary Medicine. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
ALTERNATE PATHWAYS OF STEROID BIOSYNTHESIS AND THE ORIGIN, METABOLISM, AND BIOLOGICAL EFFECTS OF RING B UNSATURATED ESTROGENS
TABLE OF CONTENTS
I. Introduction
II. Classical Pathway of Steroidogenesis
A. Source of Carbon Atoms of Cholesterol
B. Biosynthesis of Mevalonic Acid
C. Formation of Isopentenyl Pyrophosphate from Mevalonic Acid
D. Formation of Squalene from lsopentenyl Pyrophosphate
E. Cyclization of Squalene to Lanosterol
F. Conversion of Lanosterol to Cholesterol
G. Conversion of Cholesterol to Pregnenolone
III. Alternate Pathways of Steroidogenesis
A. Direct Conversion of Desmosterol to Steroid Hormones
B. Sesterterpene Pathway of Steroidogenesis
C. Biosynthesis of Ring B Unsaturated Estrogens
1. Metabolism of 7-3H-Dehydroepiandrosterone and 4-14C-Androstenedione Injected into the Umbilical Vein
2. Metabolism of 1-14C-Sodium Acetate and 7-3H-Cholesterol Injected into the Fetal Circulation
3. Metabolism of 14C-Squalene and 14C-Mevalonic Acid Injected into the Umbilical Circulation
4. Metabolism of 1-14C-Isopentenyl Pyrophosphate and (4,8,12)-14C-Farnesyl Pyrophosphate Injected into the Fetus
5. Theoretical Discussion on Alternate Pathways of Steroidogenesis
IV. Biologic Activity of Ring B Unsaturated Estrogens
V. Bioassays
A. General
B. Animal Bioassay Studies
1. Allen-Doisy Test
2. Uterotrophic Assay
3. Chick Oviduct Assay
C. Human Studies
1. Specific Responses
2. Other Responses in the Human
a. Hypothalamus and Pituitary
b. Clotting Mechanism
c. Metabolic Changes
d. Osteoporosis
e. Atherosclerosis
f. Neoplasms of the Reproductive Organs
D. The Metabolism of Equilin in Man
1. Intravenous Equilin Sulfate
2. Intravenous 3H-Equilin
VI. Concluding Remarks
Acknowledgments
References
I. INTRODUCTION
For the past 25 years, it has been generally accepted that cholesterol is the obligatory precursor in the biosynthesis of all steroid hormones. Details of the various steps for the synthesis of cholesterol from acetate and for its conversion to steroids were established mainly by in vitro incubation procedures using tissues such as the ovary, testis, and adrenal cortex. Although it became firmly established that acetate was the initial two carbon source of cholesterol, some experimentation suggested that cholesterol may not be the only precursor from which all steroid hormones are formed.
This chapter will discuss the evidence for the steroidogenic pathway which bypasses cholesterol. It will be referred to as the alternate pathway of steroidogenesis, while that which utilizes cholesterol as an obligatory intermediate will be termed the classical pathway of steroidogenesis. Although it is not the purpose of this chapter to discuss the classical pathway of biosynthesis and metabolism of steroid hormones, its salient features will be reviewed briefly to facilitate comparison with the alternate pathway, which will be discussed in detail. The metabolism and biological action of the ring B unsaturated estrogens will also be presented.
II. CLASSICAL PATHWAY OF STEROIDOGENESIS
The various steps in the classical pathway of steroidogenesis from acetate are acetate → mevalonic acid → isopentenyl pyrophosphate → 3,3-dimethyl allyl pyrophosphate → geranyl pyrophosphate → farnesyl pyrophosphate → squalene → lanosterol → cholesterol → pregnenolone → steroid hormones. The details are discussed below.
A. Source of Carbon Atoms of Cholesterol
Studies by Bloch, Lynen, Cornforth, Popják, and their colleagues on cholesterol biosynthesis from either methyl or carboxyl 14C-labeled acetate established that all 27 carbon atoms of cholesterol are derived from acetate. These investigations have been reviewed by Bloch.1 The distribution of the 15 methyl and 12 carboxyl carbons in cholesterol is shown in Figure 1. This arrangement of the carbon atoms of acetate in cholesterol follows the “isoprene rule,” which was defined by Ruzicka as a working hypothesis as early as 1921.2
B. Biosynthesis of Mevalonic Acid
Three molecules of acetic acid in the form of acetyl-CoA (coenzyme A) thioester condense to form mevalonic acid (Figure 2). All of the intermediates involved are bound to coenzyme A, and the required enzymes are present in both the cytoplasmic particles and the soluble fraction of mammalian and avian liver3, 4, 5, 6 and in yeast.7, 8 The first step, catalyzed by a soluble enzyme β-keto-thiolase, results in the formation of acetoacetyl-CoA. In the presence of 3-hydroxy-3-methyl-glutaryl-CoA synthase (which is located in the mitochondria and the soluble fraction of liver9, 10 but not in the microsomal fraction as had been reported previously11, 12), the acetoacetyl-CoA condenses with a third molecule of acetyl-CoA to give 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA), the immediate precursor of mevalonic acid. The reduction of this precursor to mevalonic acid is catalyzed by mevalonate: NADP+ oxidoreductase (EC 1.1.1.34), which is present in both yeast mitochondria13 and in the mammalian liver microsomes.14, 15 This microsomal system is thought to be the rate-limiting step in the biosynthesis of cholesterol in mammalian liver.12, 16, 17 Although this scheme is generally believed to be the major pathway of mevalonate biosynthesis, an alternate minor pathway involving malonyl-CoA has been described by Brodie et al.18, 19 The mechanisms involved in these transformations have been recently reviewed by Beytia and Porter.20
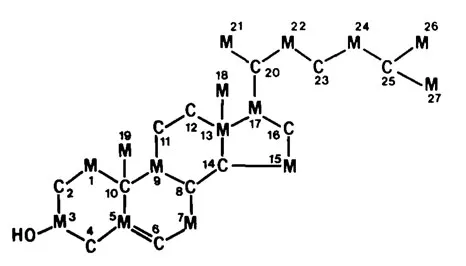
FIGURE 1. Distribution of carboxyl(C) and meth...