
eBook - ePub
Green Chemistry and Sustainable Technology
Biological, Pharmaceutical, and Macromolecular Systems
Satish A. Dake, Ravindra S. Shinde, Suresh C. Ameta, A. K. Haghi, Satish A. Dake, Ravindra S. Shinde, Suresh C. Ameta, A. K. Haghi
This is a test
Share book
- 318 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Green Chemistry and Sustainable Technology
Biological, Pharmaceutical, and Macromolecular Systems
Satish A. Dake, Ravindra S. Shinde, Suresh C. Ameta, A. K. Haghi, Satish A. Dake, Ravindra S. Shinde, Suresh C. Ameta, A. K. Haghi
Book details
Book preview
Table of contents
Citations
About This Book
Taking an interdisciplinary approach, this new volume brings together innovative research, new concepts, and novel developments in the application of new tools in green chemistry and sustainable technology. The diverse coverage includes chapters on ionic liquids as green solvents, an environmentally friendly approach to the synthesis and biological evaluation of ?-aminophosphonate derivatives, the application of nanotechnology in biological sciences and green chemistry, eco-friendly polymers, the effect of global warming and greenhouse gases on environmental system, and more.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Green Chemistry and Sustainable Technology an online PDF/ePUB?
Yes, you can access Green Chemistry and Sustainable Technology by Satish A. Dake, Ravindra S. Shinde, Suresh C. Ameta, A. K. Haghi, Satish A. Dake, Ravindra S. Shinde, Suresh C. Ameta, A. K. Haghi in PDF and/or ePUB format, as well as other popular books in Scienze biologiche & Scienza generale. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
Ionic Liquid as Green Solvents
1 PAHER University, Udaipur–313003, Rajasthan, India
2 Department of Chemistry, J. R. N. Rajasthan Vidyapeeth (Deemed to be University), Udaipur–313001, Rajasthan, India
ABSTRACT
To date, most of the chemical reactions are carried out in molecular solvents, which are many time toxic to the environment. Recently, a new class of solvent has emerged as ionic liquids (ILs). The term ILs cover one of the broadest classes of chemical compounds of salt type with low melting temperature and appreciable wide liquid range. In recent years, ILs has become mainstream solvents in different fields of chemistry. They have attracted quite justifiably, enormous attention as media for green synthesis. This has been possible owing to environmentally advantageous properties and possibility of adaptation of their structure to a specific task. In many chemical reaction processes, ILs are suggested as solvents, catalysts, reagents, or combinations of these. Due to ecofriendly, behavior, and easy design, ILs will replace traditional organic solvents. A wide variety of anions and cations are available and as such, solvent properties can be fine-tuned. A wide range of reactions have already demonstrated in this medium. ILs are being investigated for both; the dissolution of a variety of biomaterials and for their processing into higher-value products. The ability of ILs to dissolve and stabilize enzymes, proteins, DNA, and RNA is also extremely valuable in biotechnological applications. A small introduction of ILs, properties, and their applications in synthesis, extraction, and biocatalysis has been presented here.
1.1 INTRODUCTION
Solvents are a necessary part of chemical reactions. There are many industries like agrochemical, pharmaceutical, dye, pulp, paper, chemical, food, automotive, etc., which are regularly using volatile organic solvents. The main disadvantage of using an organic solvent is its vapor pressure at room temperature (RT), which helps it to be released to the environment. Therefore, they contribute strongly to the pollution (air, water) as a major source in the chemical industries. Therefore, there is a difficulty in recycling such solvents and they can directly have adverse health effects. Thus, there is an urgent need to search for alternative solvents, which are green chemical in nature and ecofriendly.
Efforts are made to develop new cleaner and greener technologies with production of less amount of waste, which are relatively less harmful to the environment also. In recent years, ionic liquid (IL) has been considered as a promising candidate, which can be definitely used as alternatives to toxic VOCs. ILs have advantages because of their unique and favorable physical properties. These are summarized in Figure 1.1. Therefore, typical ILs cations can be applied in synthesis, biocatalysis, extraction, energy storage, etc. These solvents can easily recycle a number of times.
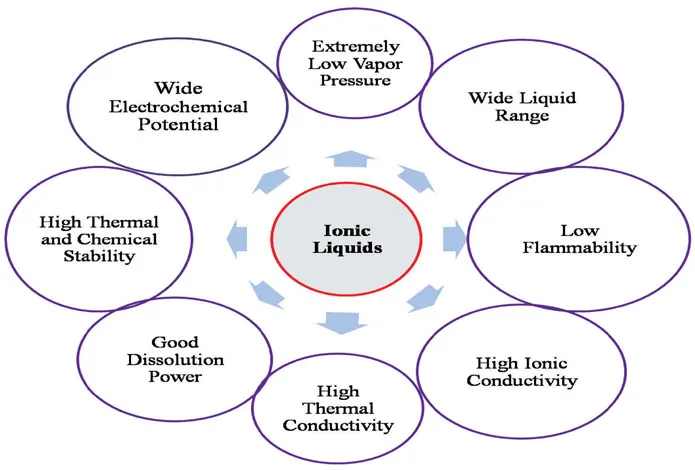
FIGURE 1.1 Physical properties of ionic liquids.
The first IL was reported in the mid-19th century, when a separate ‘red oil’ phase was formed during Friedel-Crafts reactions [1]. This separated red oil was thought to be salt, but it was identified as a stable intermediate complex formed through Friedel-Crafts reactions as evident from NMR spectroscopy [2]. Synthesis of the first IL comes to light shortly, just at the start of the 20th century, when ethyl ammonium nitrates (EtNH3); (NO3) was synthesized with a melting point of only 12°C [3]. The extraordinarily remarkable property of ILs is that such salts are liquids at or close to RT.
Most of the ILs are composed by two parts:
- First is a large massive unsymmetrical cation; and
- Second is smaller equally shaped anion.
ILs are not treated as common salts. Melt down ILs have lesser lattice energies due to incapacity to form efficient ion-ion packing and as a result, electrostatic interactions are reduced. They have reduced columbic interactions due to the diffuse charge present in the unsymmetrical cation attached with the weakly coordinating anion. ILs have low melting points, because of mainly two factors. These are:
- Weak electrostatic forces; and
- Delocalized diffuse charge.
Due to this unique property, interest in ILs is developing regularly.
ILs are mainly used as solvents and their solubility in various reagents can be tuned by selecting a particular anion or cation. ILs are now commonly used as alternative eco-friendly solvents due to large potential of combinations of various cations and anions. Their solubility also varies in chemical reactions. These are harmless and more environmentally friendly solvents. Most of synthesized ILs are miscible in water but some of them are hydrophobic also. They are excellent and alternate solvents for replacing more harmful volatile organic compounds (VOCs). ILs have four important properties. Such as:
- Rate of reaction is improved;
- Yield is increased;
- Isolation of product is improved; and
- Reduced formation of by products.
Use of ILs are based on the twelve basic principles of green chemistry, which were proposed by Clark and Macquarie in 2002 [4].
1.2 NOMENCLATURE OF IONIC LIQUIDS (ILS)
Because many ILs are composed of complex organic molecules, short names have been developed to name ILs (Figure 1.2).
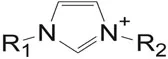
FIGURE 1.2 Imidazolium cation.
Here, 1 and 2 refer to the first letter name of the R1 and R2 carbon chain. e.g., butyl, methyl imidazolium is abbreviated to bmim. For quaternary ammonium systems, a system of the type N 1234+ is used, where the subscripts indicate the chain length of each of the four alkyl chains of the quaternary ammonium cation (Figure 1.3).
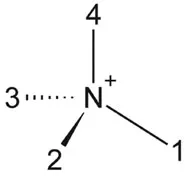
FIGURE 1.3 Quaternary ammonium cation (N1234+ = methylethylpropylbutylammonium).
ILs are composed of large unsymmetric imidazolium or quaternary ammonium systems. Comparatively, small, and symmetric ions do not form RTILs. These cations are coupled with large anions, which can either be symmetric, such as hexafluorophosphate [PF6-] or tetrafluoroborate (BF4)-, or less symmetric, such as bis(trifluoromethanesulfonyl) imide [TFSI-] or amides.
TFSI- anion forms ILs with many cations, excluding the small symmetric quaternary ammonium [R4N+] systems. However, the asymmetric amide, TSAC-, [CF3CONSO2CF3-] can form RTILs with even small symmetric quaternary ammonium ions such as tetraethyl ammonium cation, [N 2222]+ [5]. These so-called second-generation RT ILs considerably widened the range of applications, where these ILs could be applied. This is because of the fact that these not only share the advantages of their precursors, but also remain stable, when exposed to water and air [6–8].
These systems include salts based upon poorly nucleophilic anions such as [BF4̄], [PF6̄], [CF3CO2̄], [CF3SO3̄], etc. Low melting point salts, established on other cations such as complex polycationic amines, [9] and hetero-cyclic containing drugs, have also been prepared [10, 11]. Most commonly used ILs are based around the 1-alkyl-3-methylimidazolium cation, (Figure 1.4) especially the 1-ethyl-3-methylimidazolium, [emim]+ and N-butyl pyridinium (Nbupy)+ cations.
![FIGURE 1.4 Structure of 1-alkyl-3-methylimidazolium [Cnmim]+ and N-butyl pyridinium (N bupy)+ cation.](https://book-extracts.perlego.com/1584280/images/fig1_4-plgo-compressed.webp)
FIGURE 1.4 Structure of 1-alkyl-3-methylimidazolium [Cnmim]+ and N-butyl pyridinium (N bupy)+ cation.
Imidazolium and pyridinium based ILs are the most versatile used ILs due to their low volatility, good thermal stability, negligible vapor pressure, wide liquid range, electrolytic conductivity, low viscosity, etc [12–14]. They are used as both; solvents and catalysts [15–17].
1.3 SYNTHESIS OF IONIC LIQUIDS (ILS)
The process of synthesizing ILs can be divided in two sections. These are:
- Formation of the preferred cation; and
- Anion exchange.
These two methods are commonly used to obtain desired ILs by changing cation and anion. Many cations are available on commercially basis; thus, requiring only the anion exchange. There are two simple methods for the synthesis of ILs and these are quaternization and anion exchange reactions [18].
- Quaternization Reactions: It is also called alkylation process. Here, an amine or a phosphine derivative is stirred and heated with the haloalkane. This process has the benefits like commercially available low cost haloalkanes are utilized and it does not require extra utility. In this process, minimum by products are formed. Apart from this, usually, minimum temperature and time are required. The reaction kinetics is greatly dependent on the haloalkane used. The reactivity of the haloalkane decreases on increasing alkyl chain length. Usually, this reaction is carried out without using other solvents, as the reagents are normally liquids and miscible. The final desired halide salts are generally not soluble in the starting materials and these...