
eBook - ePub
Aluminium Design and Construction
John Dwight
This is a test
Share book
- 320 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Aluminium Design and Construction
John Dwight
Book details
Book preview
Table of contents
Citations
About This Book
Provides a practical design guide to the structural use of aluminium. The first chapters outline basic aluminium technology and the advantages of using aluminium in many structural applications. The major part of the book deals with structural design and presents very clear guidance for designers, with numerous diagrams, charts and examples.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Aluminium Design and Construction an online PDF/ePUB?
Yes, you can access Aluminium Design and Construction by John Dwight in PDF and/or ePUB format, as well as other popular books in Architecture & Méthodes et matériel d'architecture. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
About aluminium
1.1 GENERAL DESCRIPTION
1.1.1 The element
Aluminium is a metallic element having the chemical symbol Al, with atomic number 13 and atomic weight 27. The nucleus of the atom contains 13 protons and 14 neutrons (a total of 81 quarks). Aluminium is the third most common element in the earth’s crust, coming after oxygen and silicon. It makes up 8% of the crust’s total mass and is the most abundant metal.
1.1.2 The name
Since birth it has been dogged with a long inconvenient name (actually from the prenatal stage). And it suffers in having two different versions in common use: the N. American aluminum and the European aluminium. The name was coined by Sir Humphry Davy in about 1807 (based on a Latin word alumen), although at that stage the element did not actually exist in metallic form. Davy’s proposal was the shorter word (aluminum), but by the time commercial production began in the 1850s the extra ‘i’ had crept in. The two versions have co-existed to this day.
One wonders why the industry has done nothing to replace its four or five-syllable encumbrance with a simple user-friendly name, like the monosyllables enjoyed by other common metals. A step in the right direction would be to adopt the N. American version (‘aloominum’) worldwide, since this takes half as long to say as the European one. Better still would be to move to a snappier word altogether. The abbreviation ‘alli’ is often used in speech; why not adopt this as the official name, or even just ‘al’? Charles Dickens expressed just such a sentiment back in 1856 when he wrote:
Aluminum or as some write it, Aluminium, is neither French nor English; but a fossilised part of Latin speech, about as suited to the mouths of the populace as an icthyosaurus cutlet or a dinornis marrow-bone. It must adopt some short and vernacular title.
1.1.3 The industrial metal
It is only since 1886 that aluminium has been a serious industrial metal, that being the year when the modern smelting process was invented. It has thus been available for a very short time, compared to the thousands of years that we have had bronze, copper, lead, iron, etc. Today it easily leads the non-ferrous metals in volume usage. It is selected in preference to steel in those areas where its special properties (‘light and bright’) make it worth paying for. There are many applications where aluminium has found its rightful niche, and others where it is still on the way in. Current world consumption is some 20 million tonnes per year.
1.1.4 Alloys
Pure aluminium is weak, with a tensile strength ranging from about 90 to 140 N/mm 2 depending on the temper. It is employed for electrical conductors and for domestic products (such as pans, cans, packaging), but for serious structural use it has to be strengthened by alloying. The strongest alloys have a tensile strength of over 500 N/mm2.
There are around ten basic alloys in which wrought material (plate, sheet, sections) is produced. Unfortunately, each of these alloys appears in a vast range of different versions, so that the full list of actual specifications is long. The newcomer therefore finds material selection less simple that it is in structural steel, and there is also the alloy numbering system to contend with.
In engineering parlance the term aluminium (or aluminum) covers any aluminium-based material, and embraces the alloys as well as the pure metal. To refer specifically to the pure or commercially pure material, one has to say ‘pure aluminium’.
1.1.5 Castings
Aluminium is eminently suitable for casting. For larger items (such as sand-castings), aluminium is often a preferred option to cast iron. For smaller items (such as dye-castings), it provides a strong alternative to zinc. A wide range of casting alloys is available, different from the wrought alloys. The reliability that is possible with aluminium castings is demonstrated by the fact that they have become standard for car wheels.
1.1.6 Supposed health risk
For many years it was believed that aluminium was entirely non-toxic, and superior in this respect to most other metals. In the 1980s this picture was reversed when researchers claimed to show that the prolonged use of aluminium saucepans could cause minute amounts of the metal to be absorbed in the brain, and that this in turn could increase the risk of Alzheimer’s disease (a type of senile dementia). Following coverage in the media, aluminium cookware quickly went out of fashion and many people became fearful of aluminium in any form.
Later work has cast serious doubt on this finding, and modern opinion is that the ‘aluminium theory’ for the increased risk of Alzheimer’s disease was grossly overstated. There are two questions. First, do we really absorb significant quantities of aluminium from saucepans, relative to what we take in anyway from our food and drink? Second, at what level of absorption does aluminium become harmful? The answer to the first question is: No, because of the tenacious oxide film always present on aluminium. The answer to the second is: Nobody knows, In any case there is no need to fear aluminium outside the culinary scene.
1.1.7. Supposed fire risk
Three of the British warships sunk in the Falklands war of 1981 had aluminium superstructures. At the time, the press stated that in the severe fires preceding these sinkings the aluminium had actually burnt. This was completely untrue. The aluminium structures lost strength and distorted, but did not burn. Aluminium sections, plate, sheet, foil and wire will not support combustion. Only in the form of very finely divided powder or flake can the metal be made to burn, as can finely divided steel. Magnesium is a different story.
1.2 PHYSICAL PROPERTIES
Below we quote the physical properties of aluminium that are of interest in design, the values for the alloys being close to those for the pure metal in most cases. More precise values appear in The Properties of Aluminium and its Alloys issued annually by the Aluminium Federation in Birmingham, UK [1].
(a) Weight
The density r of pure aluminium compares as follows with steel:
Pure aluminium =2.70 g/cm3 Structural steel =7.9 g/cm3
and the value for the alloys used for wrought products lies in the range 2.67-2.80 g/cm3. A rounded value of 2.7 g/cm3 is normally used in design, leading to the following practical formulae:
See Table
where A is the section area (mm2) and t the plate thickness (mm). Table 1.1 compares aluminium with other metals.
(b) Elastic constants
Aluminium is a springy metal with a relatively low modulus of elasticity (E). For the pure metal at room temperature it compares with steel as follows:
Pure aluminium E=69 kN/mm 2
Structural steel E=205 kN/mm 2
Structural steel E=205 kN/mm 2
while the value for wrought alloys lies in the range 69–72 kN/mm. For design purposes British Standard BS.8118 adopts a standard figure of E=70 kN/mm2, which is similar to that for glass. For aluminium E decreases steadily with temperature, dropping to 67 kN/mm 2 at 100°C and 59 kN/mm2 at 200°C Poisson’s ratio (v) is higher than the accepted figure of 0.30 used for steel and should be taken equal to 0.33, based on the work of Baker and Roderick at Cambridge in 1948 [2].
The corresponding figure for the shear modulus (G), based on the above values of E and v, is:
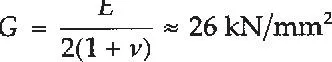
(c) Thermal expansion
The coefficient of linear expansion a for pure aluminium at room temperature compares with steel as follows:
Table 1.1 Density of metallic elements
Pure aluminium a=23.510-6/°C
Structural steel a=1210-6/°C
Structural steel a=1210-6/°C
with the value for the wrought alloys lying in the range 22–24.510-6. British Standard BS.8118 adopts a rounded value of 2310-6/°C for use in design. Note that a increases steadily with temperature, going up to 2610-6 at 200°C.
(d) Melting point
This is 660°C for pure aluminium compared with 1500°C for mild steel, while the values for the alloys are somewhat lower. The boiling point is 1800°C.
(e) Thermal constants
Aluminium is a valid material for use in heat exchangers as an alternative to copper, the thermal conductivity of the pure metal at room temperature being 240 W/m°C (about four times the figure for steel). However, the conductivity is drastically reduced by alloying, down 50% for some alloys. The specific heat of pure aluminium at room temperature is 22 cal/g°C (about twice the steel value).
(f) Electrical conductivity
Pure aluminium competes with copper in some electrical applications, and is the standard material for the conductors in overhead transmission lines (with a steel strand up the middle). The resistivity of very pure aluminium is 2.7 W cm at room temperature. Again the value is highly sensitive to alloying and is twice the above value for some alloys.
1.3 COMPARISON WITH STEEL
Wrought aluminium in its alloyed form is a strong ductile metal and has much similarity to structural steel. Its mechanical properties tend to be inferior to those of steel, the stronger alloys being comparable in strength but less ductile. The approach to structural design is much the same for the two metals and below we concentrate on the differences. Unlike steel, aluminium is, of course, non-magnetic.
1.3.1. The good points about aluminium
Lightness
Aluminium is light, one third the weight of steel.
Non-rusting
Aluminium does not rust and can normally be used unpainted. However, the strongest alloys will corrode in some hostile environments and may need protection.
This technique, the standard way of producing aluminium sections, is vastly more versatile than the rolling procedures in steel. It is a major feature in aluminium design.
Extrusion process
Weldability
Most of the alloys can be arc welded...