
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
A unique overview of the different kinds of chemical bonds that can be found in the periodic table, from the main-group elements to transition elements, lanthanides and actinides. It takes into account the many developments that have taken place in the field over the past few decades due to the rapid advances in quantum chemical models and faster computers. This is the perfect complement to "Chemical Bonding - Fundamentals and Models" by the same editors, who are two of the top scientists working on this topic, each with extensive experience and important connections within the community.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access The Chemical Bond by Gernot Frenking,Sason Shaik in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
Chemical Bonding of Main-Group Elements
Martin Kaupp
1.1 Introduction and Definitions
Prior to any meaningful discussion of bonding in main-group chemistry, we have to provide a reasonably accurate definition of what a main-group element is. In general, we assume that main-group elements are those that essentially use only their valence s- and p-orbitals for chemical bonding. This leads to a number of borderline cases that require closer inspection. Assuming that the outer d-orbitals, that is, those with principal quantum number n equal to the period in question, are not true valence orbitals (see discussion of outer d-orbital participation in bonding later in the text), we may safely define groups 13–18 as main-group elements. Group 1 is also reasonably assigned to the main groups, albeit under extreme hydrostatic pressures it appears that the elements K, Rb, and Cs turn from ns1 metals into transition metals and use predominantly their inner (n−1) d-orbitals for bonding [1]. However, sufficiently high pressures may change fundamental bonding in many elements and compounds [2]. We disregard here such extreme pressure conditions and count group 1 in the main groups.
Matters are less straightforward for group 2: whereas Be and Mg utilize only their s- and p-orbitals, Ca, Sr, Ba, and Ra use their inner (n−1) d-orbitals predominantly in covalent bonding contributions when sufficiently positively charged, as we discuss in the following text [3]. This leads to a number of peculiar structural features that bring these elements into the realm of “non-VSEPR d0 systems” that encompass early transition elements and even lanthanides, and they have also been termed “honorary d-elements” [4]. The heavy group 2 elements are nevertheless usually placed with the main groups, and it seems appropriate to include the discussion of these interesting features in the last section of this chapter.
It thus remains to discuss the inclusion of groups 11 and 12. The group 11 elements Cu, Ag, Au, and Rg clearly have a too pronounced involvement of their (n −1) d-orbitals in bonding, even in their lower oxidation states, to be safely considered main-group elements. The group 12 elements Zn, Cd, Hg, and Cn are usually considered to be main-group or “post-transition” elements. Yet recently quantum-chemical predictions [5] of oxidation-state Hg(+IV) in the form of the molecular tetra-fluoride have been confirmed by low-temperature matrix-isolation IR spectroscopy [6]. This molecule is clearly a low-spin square-planar d8 complex and thus a transition-metal species. Other, less stable Hg(+IV) compounds have been examined computationally [7], and calculations predict that the tetra-fluoride should be yet more stable for Cn (eka-Hg, element 112) [8]. However, in the largest part of the chemistry of these two elements, and in all of the accessible chemistry of Zn [9] and Cd, d-orbital participation in bonding is minor. We note in passing that Jensen [10] has vehemently opposed assignment of mercury to the transition metals based on the “extreme conditions” of the low-temperature matrix study of HgF4. The present author disagrees with this argument, as the role of the matrix is only to separate the HgF4 molecules from each other and to thus prevent aggregation and stabilization of HgF2. The low temperature is admittedly needed for entropic reasons. It is large relativistic effects that render the borderlines between the later d-elements and the main groups fuzzy in the sixth and seventh period. We nevertheless agree that the oxidation state +IV of Hg or Cn is an exception rather than the rule in group 12 chemistry, and thus discussion of the more “regular” bonding in group 12 belongs to this chapter.
If d-orbital participation in bonding is absent or at least an exception, main-group bonding may be considered simpler than that for transition-metal and f-element species. Although this is true from a general viewpoint, the variety of unusual bonding situations in main-group chemistry is nevertheless fascinating, ranging from the more common localized situations encountered in organic chemistry to delocalized bonding in, for example, electron-poor borane clusters or electron-rich noble-gas compounds to situations with even more complicated bonding-electron counts, for example, for radical-ion species or the bonding situations that may be envisioned for amorphous carbon [11]. We observe that the relative sizes of the valence s- and p-orbitals crucially influence periodic trends, and that hybridization is a more complicated matter than usually considered. In this chapter we focus mostly on general aspects and periodic anomalies, providing a basis for more specific discussions in some of the other chapters of this book.
1.2 The Lack of Radial Nodes of the 2p Shell Accounts for Most of the Peculiarities of the Chemistry of the 2p-Elements
The eigenfunctions of a Hermitean operator form a (complete) orthonormal set. This seemingly abstract mathematical condition is fundamental for the presence of nodes in wave functions. The nodes are needed to ensure orthogonality of the, exact or approximate, solutions of the Schrödinger equation. From the simplest examples like a particle in a box (closely related to the nodal characteristics of valence orbitals of extended π-systems), it is a short way to the radial and angular eigenfunctions of the hydrogen atom, which also define qualitatively the nodal structure of the atomic orbitals (AOs) of many-electron atoms. Orthogonality of AOs may be ensured either via the angular part (determined by the quantum numbers l and ml) or via the radial part (determined by quantum number n). AOs with different angular momentum are generally orthogonal via their angular part. But valence (and outer core) AOs with increasing principal quantum number n develop nodes in their radial part to stay orthogonal to inner orbitals (with lower n) of the same angular momentum. Although this is exactly true only for the isolated atom, where n, l, and ml are good quantum numbers, it also carries over approximately to atoms in molecules, with strong consequences for chemistry. The introduction of radial nodes by this “primogenic repulsion” [12] (a term indicating the necessity of staying orthogonal to the inner shells) moves the outer maximum of a radial wave function successively outwards, which makes it more diffuse. At the same time, we have to recall that the radial solutions of the Schrödinger equation for the hydrogen atom may also be written as an effective differential equation of a particle in an l-dependent potential. This effective potential contains a repulsive term due to centrifugal forces for l ≥ 1 but not for l = 0. As a consequence, s-orbitals have finite amplitude at the nucleus, whereas p-, d- or f-orbitals vanish at the nucleus (within a nonrelativistic framework). However, most importantly for the present discussion, the repulsive centrifugal term moves the outer maximum of the p-orbitals to larger radii. Therefore, np-orbitals tend to be larger and more diffuse than the corresponding ns-orbitals, with the major exception of n = 2 – because of the lack of a radial node, the 2p-orbital is actually similar in size to the 2s-orbital, which has one radial node to stay orthogonal to the 1s-shell [13]. The approximate relative sizes of the valence s- and p-orbitals in period 2 and 3 many-electron atoms from relativistic Hartree–Fock calculations are shown in Figure 1.1. On average, the 2p-orbitals are less than 10% larger than the 2s-orbitals, whereas the 3p-orbitals exceed the 3s-orbitals by 20–33%. Differences increase further down a given group (modified by spin-orbit coupling for the heavier elements) [13].
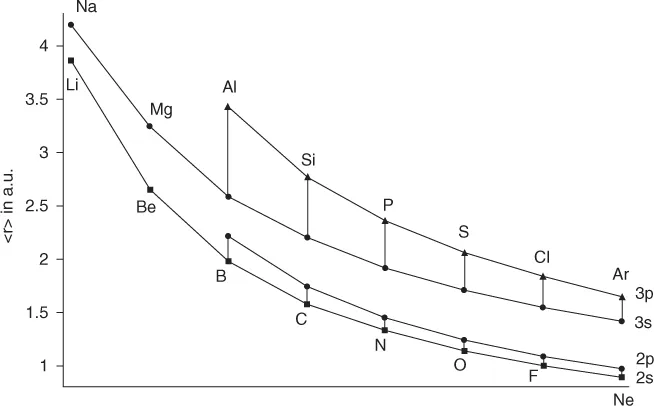
Fig...
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- List of Contributors
- Chapter 1: Chemical Bonding of Main-Group Elements
- Chapter 2: Multiple Bonding of Heavy Main-Group Atoms
- Chapter 3: The Role of Recoupled Pair Bonding in Hypervalent Molecules
- Chapter 4: Donor–Acceptor Complexes of Main-Group Elements
- Chapter 5: Electron-Counting Rules in Cluster Bonding – Polyhedral Boranes, Elemental Boron, and Boron-Rich Solids
- Chapter 6: Bound Triplet Pairs in the Highest Spin States of Monovalent Metal Clusters
- Chapter 7: Chemical Bonding in Transition Metal Compounds
- Chapter 8: Chemical Bonding in Open-Shell Transition-Metal Complexes
- Chapter 9: Modeling Metal–Metal Multiple Bonds with Multireference Quantum Chemical Methods
- Chapter 10: The Quantum Chemistry of Transition Metal Surface Bonding and Reactivity
- Chapter 11: Chemical Bonding of Lanthanides and Actinides
- Chapter 12: Direct Estimate of Conjugation, Hyperconjugation, and Aromaticity with the Energy Decomposition Analysis Method
- Chapter 13: Magnetic Properties of Aromatic Compounds and Aromatic Transition States
- Chapter 14: Chemical Bonding in Inorganic Aromatic Compounds
- Chapter 15: Chemical Bonding in Solids
- Chapter 16: Dispersion Interaction and Chemical Bonding
- Chapter 17: Hydrogen Bonding
- Chapter 18: Directional Electrostatic Bonding
- Index
- End User License Agreement