
Lithium Batteries
Advanced Technologies and Applications
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Lithium Batteries
Advanced Technologies and Applications
About this book
Explains the current state of the science and points the way to technological advances
First developed in the late 1980s, lithium-ion batteries now power everything from tablet computers to power tools to electric cars. Despite tremendous progress in the last two decades in the engineering and manufacturing of lithium-ion batteries, they are currently unable to meet the energy and power demands of many new and emerging devices. This book sets the stage for the development of a new generation of higher-energy density, rechargeable lithium-ion batteries by advancing battery chemistry and identifying new electrode and electrolyte materials.
The first chapter of Lithium Batteries sets the foundation for the rest of the book with a brief account of the history of lithium-ion battery development. Next, the book covers such topics as:
- Advanced organic and ionic liquid electrolytes for battery applications
- Advanced cathode materials for lithium-ion batteries
- Metal fluorosulphates capable of doubling the energy density of lithium-ion batteries
- Efforts to develop lithium-air batteries
- Alternative anode rechargeable batteries such as magnesium and sodium anode systems
Each of the sixteen chapters has been contributed by one or more leading experts in electrochemistry and lithium battery technology. Their contributions are based on the latest published findings as well as their own firsthand laboratory experience. Figures throughout the book help readers understand the concepts underlying the latest efforts to advance the science of batteries and develop new materials. Readers will also find a bibliography at the end of each chapter to facilitate further research into individual topics.
Lithium Batteries provides electrochemistry students and researchers with a snapshot of current efforts to improve battery performance as well as the tools needed to advance their own research efforts.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
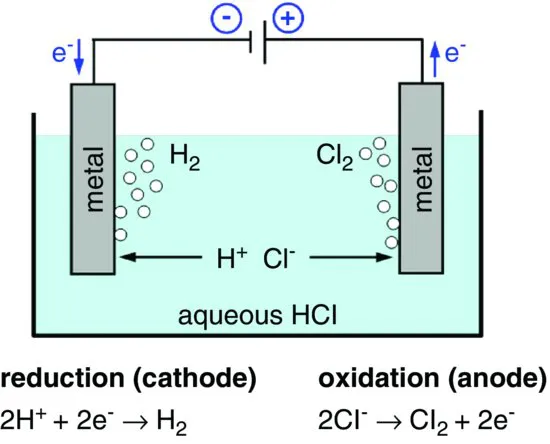


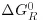
Table of contents
- Cover
- Series
- Title Page
- Copyright
- Contributors
- Preface
- Chapter 1: Electrochemical Cells: Basics
- Chapter 2: Lithium Batteries: from early stages to the future
- Chapter 3: Additives in Organic Electrolytes for Lithium Batteries
- Chapter 4: Electrolytes for Lithium-Ion Batteries with High-Voltage Cathodes
- Chapter 5: Core–Shell Structure Cathode Materials for Rechargeable Lithium Batteries
- Chapter 6: Problems and expectancy in Lithium Battery technologies
- Chapter 7: Fluorine-Based Polyanionic Compounds for High-Voltage Electrode Materials
- Chapter 8: Lithium–Air and Other Batteries Beyond Lithium-Ion Batteries
- Chapter 9: Aqueous Lithium–Air Systems
- Chapter 10: Polymer electrolytes for lithium–air batteries
- Chapter 11: Kinetics of the Oxygen Electrode in Lithium–Air Cells
- Chapter 12: Lithium-ion batteries and supercapacitors for use in hybrid electric vehicles
- Chapter 13: Li4Ti5O12 for High-Power, Long-Life, and Safe Lithium-Ion Batteries
- Chapter 14: Safe Lithiium Rechargeable Batteries Based On Ionic Liquids
- Chapter 15: Electrolytic Solutions for Rechargeable Magnesium Batteries
- Chapter 16: Rechargeable Sodium and Sodium-Ion Batteries
- Index
- The Electrochemical Society Series
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app