
Orbital Interactions in Chemistry
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Orbital Interactions in Chemistry
About this book
Explains the underlying structure that unites all disciplinesin chemistry Now in its second edition, this book explores organic, organometallic, inorganic, solid state, and materials chemistry, demonstrating how common molecular orbital situations arisethroughout the whole chemical spectrum. The authors explore therelationships that enable readers to grasp the theory thatunderlies and connects traditional fields of study withinchemistry, thereby providing a conceptual framework with which tothink about chemical structure and reactivity problems. Orbital Interactions in Chemistry begins by developingmodels and reviewing molecular orbital theory. Next, the bookexplores orbitals in the organic-main group as well as in solids.Lastly, the book examines orbital interaction patterns that occurin inorganic-organometallic fields as well as clusterchemistry, surface chemistry, and magnetism in solids. This Second Edition has been thoroughly revised andupdated with new discoveries and computational tools since thepublication of the first edition more than twenty-five years ago.Among the new content, readers will find:
* Two new chapters dedicated to surface science and magneticproperties
* Additional examples of quantum calculations, focusing oninorganic and organometallic chemistry
* Expanded treatment of group theory
* New results from photoelectron spectroscopy Each section ends with a set of problems, enabling readers totest their grasp of new concepts as they progress through the text.Solutions are available on the book's ftp site. Orbital Interactions in Chemistry is written for bothresearchers and students in organic, inorganic, solid state, materials, and computational chemistry. All readers will discoverthe underlying structure that unites all disciplines inchemistry.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1.1 Introduction
1.2 Atomic Orbitals
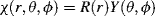
| Orbital Type | Expression for Y | |
| s | 1 | 1 |
| px | x/r | sin θ cos ϕ |
| py | y/r | sin θ sin ϕ |
| pz | z/r | cos θ |
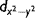 | (x2 − y2)/r2 | sin2 θ cos 2ϕ |
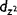 | (3z2 − r2)/r2 | 3cos2θ − 1 |
| dxy | xy/r2 | sin2 θ sin 2ϕ |
| dxz | xz/r2 | sin θ cos θ cos ϕ |
| dyz | yz/r2 | sin θ cos θ sin ϕ |
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- About the Authors
- Chapter 1: Atomic and Molecular Orbitals
- Chapter 2: Concepts of Bonding and Orbital Interaction
- Chapter 3: Perturbational Molecular Orbital Theory
- Chapter 4: Symmetry
- Chapter 5: Molecular Orbital Construction from Fragment Orbitals
- Chapter 6: Molecular Orbitals of Diatomic Molecules and Electronegativity Perturbation
- Chapter 7: Molecular Orbitals and Geometrical Perturbation
- Chapter 8: State Wavefunctions and State Energies
- Chapter 9: Molecular Orbitals of Small Building Blocks
- Chapter 10: Molecules with Two Heavy Atoms
- Chapter 11: Orbital Interactions through Space and through Bonds
- Chapter 12: Polyenes and Conjugated Systems
- Chapter 13: Solids
- Chapter 14: Hypervalent Molecules
- Chapter 15: Transition Metal Complexes: A Starting Point at the Octahedron
- Chapter 16: Square Planar, Tetrahedral ML4 Complexes and Electron Counting
- Chapter 17: Five Coordination
- Chapter 18: The C2v ML3 Fragment
- Chapter 19: The ML2 and ML4 Fragments
- Chapter 20: Complexes of ML3, MCp and Cp2M
- Chapter 21: The Isolobal Analogy
- Chapter 22: Cluster Compounds
- Chapter 23: Chemistry on the Surface
- Chapter 24: Magnetic Properties
- Appendix I: Perturbational Molecular Orbital Theory
- Appendix II: Some Common Group Tables
- Appendix III: Normal Modes for Some Common Structural Types
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app