![]()
1
Introduction
Anne Bridgen
Croft Dhu, Newtonmore, Inverness-shire, Scotland
1.1 Background
Viruses with ribonucleic acid (RNA) genomes make up many of our current most serious human pathogens. For example, influenza A virus, poliovirus, rotaviruses, dengue virus, hepatitis C virus, West Nile fever virus, yellow fever virus and measles virus are all RNA viruses, and are between them responsible for millions of human deaths each year. Rotaviruses alone are responsible for around 350,000–600,000 infant deaths each year from diarrhoea (Parashar et al., 2003). One of the features of RNA viruses is that the viral polymerase responsible for their replication is not very accurate as there is no proof-reading capacity. This low accuracy means that, in the presence of antiviral drugs, viral escape mutants soon arise which no longer respond to the drug. There are thus very few effective antivirals directed against RNA viruses. In addition, many of the new emerging viruses which arise through viral mutation, genome segment reassortment or host switching to suddenly enter the human population are RNA viruses. These include the coronavirus severe acute respiratory syndrome (SARS) virus, Ebola and Marburg filoviruses, and avian and swine flu, and are the viruses that tend to cause the highest mortality rates. There is thus a high requirement to be able to analyse these viruses and to develop effective vaccines and antivirals.
RNA viruses possess several different types of RNA genomes. Some have a non-segmented genome, or the genome can be split into a number of different segments, for example, 2 for arenaviruses, 3 for bunyaviruses, 7–8 for influenza viruses and 10–12 for reoviruses. In addition, they can comprise positive sense, negative sense or ambisense RNA, and be either single- or double-stranded. Positive sense RNAs can be translated directly into protein, while a negative sense RNA has first to be transcribed by the viral proteins to form positive stranded RNA that can be translated. Ambisense RNAs are those which contain genes running in both orientations within the same genome or genome segment. There are also retroviruses and hepadnaviruses which go through both RNA and deoxyribonucleic acid (DNA) phases via reverse transcription of their RNA. These last named groups of viruses, which include the human immunodeficiency viruses, will not be discussed in this volume despite their importance, as the amount of research in this area would easily require a separate volume.
In classical genetics, the specific genes in an organism were deduced from observations of the phenotype of the organism. Reverse genetics is a term coined to describe processes where information flows in the opposite direction, that is, the gene is determined or altered directly, and the resultant phenotype observed. In the context of virology, this then refers to changes introduced directly into the complementary DNA (cDNA) used to generate infectious RNA virus or virus-like particles, in order to study the function of specific gene sequences and proteins, and the term has come to be applied to the ability to go from a DNA copy of the viral genome to a new virus. Neumann and Kawaoka (2004) define reverse genetics as the generation of a virus entirely from cDNA. It is an incredibly powerful tool both for the generation of modified viruses, which can act as vaccines or vectors, and for the analysis of viral genes and non-coding sequences.
1.2 Reverse genetics for different classes of genome
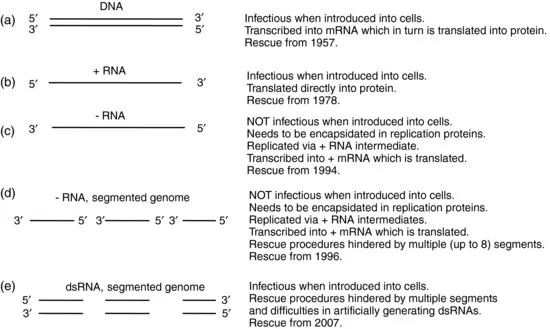
One of the most definitive ways in which to study the roles of specific sequences in viral genomes is to modify them and to generate infectious virus, that is, to ‘rescue’ the virus, from these modified sequences. For DNA viruses this was relatively straightforward once molecular biological techniques became sufficiently sophisticated to allow this, as the DNA could be introduced directly into cells to generate infectious virus. Thus, infectious T2 bacteriophage was rescued from DNA as early as 1957 (Fraser et al., 1957). The first RNA virus to be rescued from its cDNA was the bacteriophage Qbeta rescued by Taniguchi et al. (1978), while the first mammalian plus stranded RNA virus to be rescued was poliovirus by Racaniello and Baltimore (1981). Researchers subsequently discovered that this process was more efficient if the RNA was transcribed in vitro and the nascent RNA transfected into cells (Boyer and Haenni, 1994); this process was then applied to many plus sense RNA viruses. Some difficulties were encountered with specific families of viruses, however, such as coronaviruses, as is discussed in Section 1.4.
Negative sense RNA viruses proved less amenable to such studies as the minimal infectious unit comprises the viral RNA encapsidated by the nucleocapsid and replication proteins to form a ribonucleoprotein (RNP) complex. It was not until 1994 that Schnell et al. (1994) succeeded in rescuing the first negative sense RNA virus, the rhabdovirus rabies virus, from cDNA. One of the main reasons for this breakthrough was the decision to transfect cells with cDNA plasmids encoding the viral antigenome rather than the genome. This meant that there was less negative sense RNA present in the cell which could hybridise to the positive sense viral mRNAs and thus induce host innate immune responses.
To add to the difficulties of rescuing negative sense RNA viruses from cDNA, many of them comprise segmented genomes, so, for their rescue, cells must be transfected with constructs for each of the genome segments as well as for the replication proteins. Early rescue experiments for the eight-segmented genome virus influenza A virus involved modification of single RNA segments and use of helper viruses (Luytjes et al., 1989; Enami et al., 1990). However, this is not an efficient process as only a small proportion of the helper viruses acquires the novel segment. The first segmented, negative sense RNA virus to be rescued entirely from cDNA was the tri-segmented bunyavirus Bunyamwera virus (Bridgen and Elliott, 1996). This used the approach initiated for the non-segmented rabies virus in using positive sense, antigenomic constructs for rescue. Rescue of influenza A virus entirely from cDNA followed later (Fodor et al., 1999; Neumann et al., 1999), in a procedure involving transfection of cells with 12 different plasmids. This original technique has been modified extensively and now rescue can be achieved using far fewer plasmids.
Of the viruses described in this volume, the last to be rescued were the double-stranded RNA (dsRNA) genome viruses. Not only do these have genomes of dsRNA, a structure which does not naturally occur in cells and which therefore induces host innate immune responses, but also many have multiple genome segments, making the rescue more complex.
Thus, the practical way in which rescue is achieved is very different depending on the nature of the genome, as is summarised in Figure 1.1. In this volume we are showcasing the RNA viruses from each of these genomic groups, so that there are examples of what has been achieved and what the problems have been for each of these groups. To date, representatives of most of the human and animal virus families have been rescued from cDNA (see Table 1.1). There has also been an explosion of work in plant virology, which has seen a considerable number of plant pathogens rescued in the past decade. There are, however, many viruses of invertebrates and plants that have not yet been studied by this approach, and indeed many species within families in which the prototype virus has been well studied by reverse genetic technology but other members have not.
Table 1.1 Important dates in the history of human and animal virus reverse genetics.
The book also discusses viral quasispecies and the implication of this theory on approaches to reverse genetics (see Chapter 11). The theory implies that viruses are not unique, but instead comprise a swarm of inseparable and related molecules, which therefore instantly creates a problem for researchers trying to produce a single synthetic virus molecule. The practical procedures used during rescue, such as the cell type or level of plaque purification, will impact on the level of heterogeneity of the virus (Section 11.2.3) so this theory has practical application to the way in which rescue experiments are conducted.
1.3 Methodology
In this chapter we are going to consider several facets of the methodology behind current reverse genetics techniques, specifically:
1. Stages in virus reverse genetics.
2. Use of different promoters.
3. Obtaining precise genome ends.
4. Increasing rescue efficiencies.
5. Combining material from different genetic segments.
6. Confirmation of the rescue phenotypes.
1.3.1 Stages in virus reverse genetics: minigenome replication; replication of virus-like particles (VLPs) and whole virus rescue
Many researchers start their rescue experiments by using a minigenome system comprising a reporter gene bounded by viral sequences which provide the signals for viral transcription and replication. These can be replicated and transcribed in vitro by the viral replicative genes supplied from appropriate plasmids. This is a useful first stage to ensure that the cloned polymerase is functional and the replication signals are correct before attempting full virus rescue. One commonly used reporter gene used is that encoding the jellyfish Aequorea victoria Green Fluorescent Protein (GFP), which can fluoresce in the presence of ultraviolet light, as well as its spectral variants including blue, red and yellow fluorescent proteins. Luciferase genes from the firefly Photinus pyralis or the sea pansy Renilla reniformis are capable of bioluminescence in the presence of suitable substrates. Another reporter gene is that for chloramphenicol acetyl transferase (CAT). Choice of reporter depends on the cloning capacity (CAT and GFP are both quite small proteins), the application, and the predicted stability/toxicity (GFP features less well here). The reporter genes are cloned in the same sense as the viral genes. They therefore have to be transcribed into mRNA before they can be translated for negative-sense RNA viruses.
Use of the minigenome systems ensures everything is working well before full rescue is att...