![]()
CHAPTER 1
Anatomic Aspects of Bone Ossification and their Magnetic Resonance Counterparts
Guido Carpino1, Ernesto Tomei2, Richard C. Semelka3, and Eugenio Gaudio4
1Department of Motor, Human and Health Sciences, University of Rome Foro Italico, Rome, Italy
2Department of Radiology, Oncology and Anatomy Pathology, Sapienza University of Rome, Rome, Italy
3Department of Radiology, UNC School of Medicine, Chapel Hill, North Carolina, USA
4Department of Anatomical, Histological, Forensic Medicine and Orthopedic Sciences, Sapienza University of Rome, Rome, Italy
1.1 Endochondral ossification
Endochondral ossification is the process by which a bone develops from a pre-existing model composed of hyaline cartilage. It begins around the sixth week of fetal development and continues into the individual's twenties. Most bones of the body, including the vertebrae, ribs, sternum, scapula, pelvis, and bones of the limbs, develop in this way [1,2].
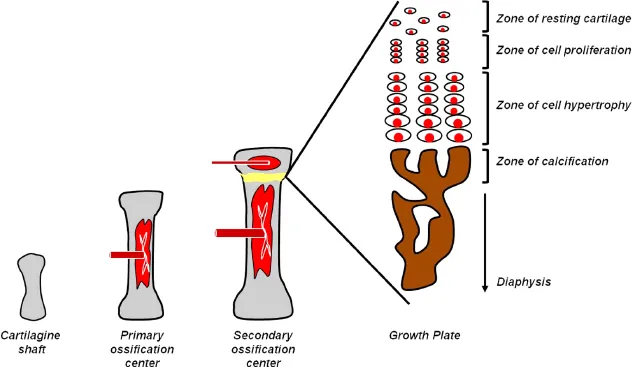
Ossification proceeds in the following fashion: the center of the cartilage model is invaded by mesenchymal stem cells which form the primary center of ossification. Later, at each end of the cartilage model, secondary centers of ossification appear (Figure 1.1). These centers of ossification gradually encroach on the remaining cartilage, ultimately replacing it completely (except at the articular surfaces) by the time skeletal maturity is reached [1,3,4]. The cartilage model is important as the source of longitudinal bone growth. The ossification of the cartilage model is a well-organized process which could be subdivided into several phases [1,3,4].
1.1.1 Primary center of ossification (fetal life)
Chondrocytes at the center of the cartilaginous model (the diaphysis) begin to increase in number and size. The chondrocytes' hypertrophy is followed by their apoptosis, matrix calcification, and vascular invasion. The blood vessels bring with them mesenchymal stem cells that will give rise both to osteoblasts and osteoclasts. The invading cells stimulate the removal of the calcified cartilage and its replacement by trabecular bone and bone marrow.
1.1.2 Growth plate
As the bone marrow cavity expands toward the epiphyses, the chondrocytes at the epiphyseal margin proliferate rapidly, forming longitudinal columns of flattened cells. Thus, the primary growth plate is interposed between the cartilaginous epiphysis and the newly generated bone (bone marrow cavity). The growth plate is composed of zones of resting, proliferative, maturing, and hypertrophic chondrocytes, and of calcification.
Zone of resting cartilage. This region, farthest from the marrow cavity, consists of typical hyaline cartilage that as yet shows no sign of transforming into bone.
Zone of cell proliferation. A little closer to the marrow cavity, chondrocytes multiply and arrange themselves into longitudinal columns of flattened lacunae.
Zone of cell hypertrophy. Next, the chondrocytes cease to divide and begin to hypertrophy.
Zone of calcification and bone deposition. Minerals are deposited in the matrix between the columns of lacunae and calcify the cartilage. Apoptosis of hypertrophic chondrocytes occurs. Each column is converted into a longitudinal channel, which is immediately invaded by blood vessels and marrow from the marrow cavity. Osteoblasts line up along the walls of these channels and begin depositing concentric lamellae of matrix, while osteoclasts dissolve the temporarily calcified cartilage.
Within the growth plate, chondrocyte proliferation (zone of cell proliferation) is balanced by chondrocyte apoptosis and the replacement of the calcified cartilage with bone (zone of bone deposition). In this way, the width of the growth plate during development is maintained while the bone increases in length [1,3,4].
1.1.3 Secondary centers of ossification (later in development)
Roughly spherical secondary centers of ossification form within the cartilaginous epiphyses. The secondary ossification center becomes hollowed out by the same process as occurs in the diaphysis, generating a secondary marrow cavity in the epiphysis. This cavity expands outward from the center, in all directions. In bones with two secondary ossification centers, one center lags behind the other in development, so at birth there is a secondary marrow cavity at one end while chondrocyte growth has just begun at the other [1].
The appearance and development of secondary centers of ossification is a well-organized process. It has been shown that this process is driven by vascular endothelial growth factors (VEGFs). Immediately prior to the vascular invasion of the epiphysis, a positive reaction to VEGF is localized in chondrocytes of the epiphyseal cartilage close to the capsule insertion. During the development and expansion of the secondary ossification center, VEGF expression is higher in chondrocytes but decreases when the epiphysis is diffusely ossified. VEGF is also expressed by mesenchymal cells present in and around the fibrous tissue where the secondary ossification center will develop [2,5].
Vascular corrosion casts confirm that vessels that penetrate into the epiphysis arise primarily from the periosteal and capsular networks, and vascular connections with the diaphyseal circulation are not evident. Hence the epiphyseal microcirculation is distinct from that of the diaphysis, and arises from the vessels present in the capsule and the periosteal networks. In young animals the only capillaries are bone marrow sinusoids and a few subchondral capillaries. In adult animals small vessels run between the clusters of sinusoids forming the trabecular circulation. Capillary sprouts from sinusoids are observed both in young and adult animals. Thus, in adults, different proper microcirculatory districts can be distinguished in the epiphysis: (i) the sinusoidal network, which supplies the hematopoiesis of the bone marrow and the adjacent osteogenic tissue, and (ii) the bone tissue microcirculation, limited to small vessels that supply the metabolism and the remodeling of bone tissue [2,5,6].
These observations demonstrate that VEGF production by chondrocytes begins a few days after birth, supports the rapid vascular growth from the surrounding soft tissues, and is chronologically related to the development of the secondary ossification centers [2,5,6].
The microvascular organization and its adaptation to epiphyseal growth represents the morphologic basis for understanding the interaction between the different tissues in developing and adult epiphyses, with most of the basic research performed on the rat model [2,5,6].
1.2 Longitudinal bone growth
Longitudinal bone growth continues until puberty when chondrocyte proliferation ceases and the primary and secondary centers of ossification fuse. A thin layer of cartilage covering the joint surface remains throughout adulthood, protecting the underlying bone and providing a smooth surface for articulation [1,3,4].
The region of transition from cartilage to bone at each end of the primary marrow cavity is called the metaphysis. By the late teens to early twenties, all remaining cartilage in the epiphyseal plate is generally consumed and the gap between the epiphysis and diaphysis closes. The primary and secondary marrow cavities then unite into a single cavity, and the bone can no longer grow in length [1,3,4].
The junctional region where they meet is filled with spongy bone, and the site of the original epiphyseal plate is marked with a line of slightly denser spongy bone called the epiphyseal line.
When the epiphyseal plate is depleted, the epiphysis has “closed” because no gap between the epiphysis and diaphysis is visible on X-ray. The epiphyseal plates close at different ages in different bones and in different regions of the same bone. The state of closure in various bones is often used in forensic science to estimate the age at death of a subadult skeleton [1,3,4].
1.3 Magnetic resonance aspects of endochondral ossification
Magnetic resonance (MR) imaging can study in detail the dynamic process of skeletal growth and maturation [7]. The bone cortex is of very low signal intensity (SI) on images obtained with all sequences (Figures 1.2 and 1.3). The periosteum can be observed as a thin, low-SI, linear structure that parallels the bone cortex; this appearance is due to its composition of collagen fibers, fibroblasts, and osteoprogenitor cells. The articular cartilage is a highly organized form of hyaline cartilage and can be observed on water-sensitive images as a thin hyperintense rim surrounding the less well-organized hyaline cartilage of the developing epiphysis (Figures 1.2 and 1.3). Moreover, it can show details on: (i) bone marrow conversion, (ii) epiphyseal cartilage, and (iii) growth plate [7].