
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Introduction to Particle Technology
About this book
This textbook provides an excellent introduction to particle technology with worked examples and exercises. Based on feedback from students and practitioners worldwide, it has been newly edited and contains new chapters on slurry transport, colloids and fine particles, size enlargement and the health effects of fine powders. Topics covered include:
- Characterization (Size Analysis)
- Processing (Granulation, Fluidization)
- Particle Formation (Granulation, Size Reduction)
- Storage and Transport (Hopper Design, Pneumatic Conveying, Standpipes, Slurry Flow)
- Separation (Filtration, Settling, Cyclones)
- Safety (Fire and Explosion Hazards, Health Hazards)
- Engineering the Properties of Particulate Systems (Colloids, Respirable Drugs, Slurry Rheology)
This book is essential reading for undergraduate students of chemical engineering on particle technology courses. It is also valuable supplementary reading for students in other branches of engineering, applied chemistry, physics, pharmaceutics, mineral processing and metallurgy. Practitioners in industries in which powders are handled and processed may find it a useful starting point for gaining an understanding of the behavior of particles and powders.
Review of the First Edition taken from High Temperatures - High pressures 1999 31 243 – 251
"..This is a modern textbook that presents clear-cut knowledge. It can be successfully used both for teaching particle technology at universities and for individual study of engineering problems in powder processing."
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Particle Size Analysis
1.1 INTRODUCTION
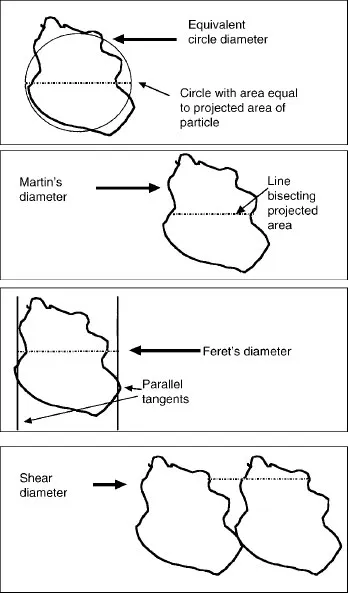
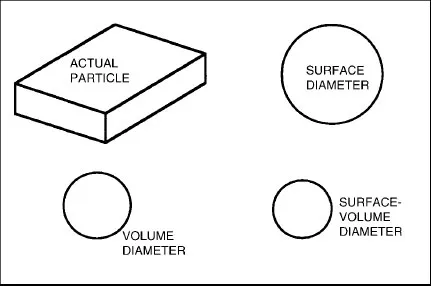
1.2 DESCRIBING THE SIZE OF A SINGLE PARTICLE

1.3 DESCRIPTION OF POPULATIONS OF PARTICLES
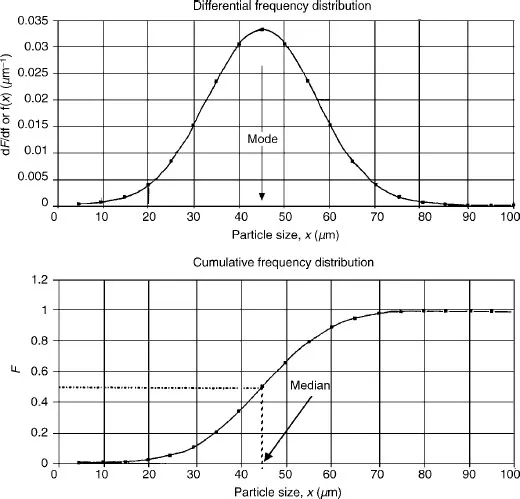
Table of contents
- Cover
- Contents
- Title page
- Copyright
- About the Contributors
- Preface to the Second Edition
- Preface to the First Edition
- Introduction
- 1 Particle Size Analysis
- 2 Single Particles in a Fluid
- 3 Multiple Particle Systems
- 4 Slurry Transport
- 5 Colloids and Fine Particles
- 6 Fluid Flow Through a Packed Bed of Particles
- 7 Fluidization
- 8 Pneumatic Transport and Standpipes
- 9 Separation of Particles from a Gas: Gas Cyclones
- 10 Storage and Flow of Powders-Hopper Design
- 11 Mixing and Segregation
- 12 Particle Size Reduction
- 13 Size Enlargement
- 14 Health Effects of Fine Powders
- 15 Fire and Explosion Hazards of Fine Powders
- 16 Case Studies
- Notation
- References
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app