
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Crystallization is a natural occurring process but also a process abundantly used in the industry. Crystallization can occur from a solution, from the melt or via deposition of material from the gas phase (desublimation). Crystals distinguish themself from liquids, gases and amorphous substances by the long-range order of its building blocks that entail the crystals to be formed of well-defined faces, and give rise to a large number of properties of the solid.
Crystallization is used at some stage in nearly all process industries as a method of production, purification or recovery of solid materials. Crystallization is practiced on all scales: from the isolation of the first milligrams of a newly synthesized substance in the research laboratory to isolating products on the mulit-million tonne scale in industry. The book describes the breadth of crystallization operations, from isolation from a reaction broth to purification and finally to tailoring product properties.
In the first section of the book, the basic mechanisms - nucleation, growth, attrition and agglomeration are introduced. It ensures an understanding of supersaturation, the driving force of crystallization. Furthermore, the solubility of the substance and its dependences on process conditions and the various techniques of crystallization and their possibilities and limitations are discussed. Last but not least, the first part includes an intensive treatment of polymorphism . The second part builds on the basics, exploring how crystallization processes can be developed, either batch-wise or continuous, from solution or from the melt. A discussion of the purification during crystallization serves as a link between the two sections, where practical aspects and an insight using theoretical concepts are combined. Mixing and its influence on the
crystallization as well as the mutual interference of down-stream processes with the crystallization are also treated. Finally, techniques to characterize the crop are discussed.
The third part of the book is dedicated to accounts of actual developments and of carried-out crystallizations. Typical pitfalls and strategies to avoid these as well as the design of robust processes are presented.
Crystallization is used at some stage in nearly all process industries as a method of production, purification or recovery of solid materials. Crystallization is practiced on all scales: from the isolation of the first milligrams of a newly synthesized substance in the research laboratory to isolating products on the mulit-million tonne scale in industry. The book describes the breadth of crystallization operations, from isolation from a reaction broth to purification and finally to tailoring product properties.
In the first section of the book, the basic mechanisms - nucleation, growth, attrition and agglomeration are introduced. It ensures an understanding of supersaturation, the driving force of crystallization. Furthermore, the solubility of the substance and its dependences on process conditions and the various techniques of crystallization and their possibilities and limitations are discussed. Last but not least, the first part includes an intensive treatment of polymorphism . The second part builds on the basics, exploring how crystallization processes can be developed, either batch-wise or continuous, from solution or from the melt. A discussion of the purification during crystallization serves as a link between the two sections, where practical aspects and an insight using theoretical concepts are combined. Mixing and its influence on the
crystallization as well as the mutual interference of down-stream processes with the crystallization are also treated. Finally, techniques to characterize the crop are discussed.
The third part of the book is dedicated to accounts of actual developments and of carried-out crystallizations. Typical pitfalls and strategies to avoid these as well as the design of robust processes are presented.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Crystallization: Introduction
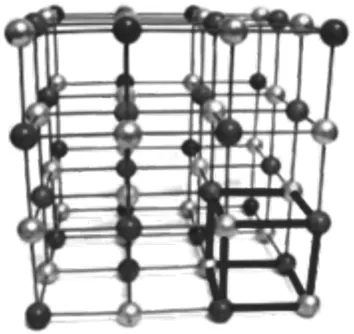
The beauty of crystals can be found in both the naturally appearing minerals such as diamonds or quartzite crystals and the industrial products such as sugar crystals. Crystals that are bound by flat faces intersecting at well-defined angels are characteristic of the substance and give the crop a reproducible appearance. This regular appearance is due to the long-range order of the building blocks of the crystal, be it either atoms or molecules. For example, in sodium chloride, the sodium and chlorine atoms are arranged in a cubic lattice (Figure 1.1). This arrangement maximizes the attractive interactions between the building blocks and thus minimizes the energetic state. The long-range order of its building blocks makes the crystalline state distinct from the gaseous and liquid as well as the amorphous solid state. The long-range order is also the root cause of a number of well-defined properties of the crystals, so these properties can be tailored through the crystallization process.
Figure 1.1 Arrangement of the sodium and chlorine atoms in the simple cubic lattice of sodium chloride.
A further consequence of the well-defined arrangement of the building blocks is the outer shape of the crystals; crystals are limited by flat faces that intersect under well-defined angles determined by the lattice. This can be easily observed for the large crystals of rock sugar (Figure 1.2). For a given substance, ordering is a characteristic. Consequently, the faces and their angles are characteristics of a given substance. All crystals grown under similar conditions will exhibit the same faces and partitioning of the faces.
Figure 1.2 Crystals of rock sugar with large well-developed flat faces, which intersect under certain angels characteristic of the substance; note that the apparent roughness of the faces arises not from the crystallization process, but from the downstream processes like washing.

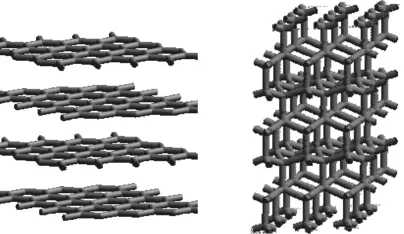
Though the lattice is characteristic of a given substance, a large number of substances can crystallize following more than one ordering motive, leading to polymorphism. Carbon, for example, can crystallize in two different lattices, as diamond and as graphite. In diamond, the carbon atoms are arranged in two face-centered cubic lattices; in graphite, the carbon atoms are arranged in layers in which the atoms have a hexagonal symmetry (Figure 1.3). With respect to energy and stability, graphite is more stable than diamond at room temperature and ambient pressure, though the barrier for transformation is extremely high.
Figure 1.3 Carbon crystallizing in two different modifications – as graphite and as diamond – having different lattice arrangements.
A further equally important consequence of packing is the well-known purification during crystallization; only molecules of one type are incorporated, while most other molecules are rejected by the growing interface. This is for geometric as well as for energetic reasons as it is energetically favorable to incorporate a proper building block instead of an impurity molecule.
Crystallization belongs to the oldest unit operations known to mankind. Namely, the crystallization of salts can be found through the ages. Early civilizations in coastal areas used large open ponds, salines, to crystallize out the salt, which could then be easily handled, stored, transported, traded, and finally used (Figure 1.4). Salines around the seaport of Ostia are said to have facilitated the development of Roma and the Roman Empire.
Figure 1.4 Solar ponds in Venezuela (courtesy of Günter Hofmann).

However, salt obtained by evaporation of seawater had a number of drawbacks; the purity was limited, mainly due to the high content of inclusions of mother liquor that entrained impurities. Hence, industrial techniques have developed over the time for the industrial crystallization of salt, resulting in the modern continuous vacuum crystallization apparatus.
Today, crystalline products can be found in every aspect of life. Relevant product properties are determined by crystal properties and thus tailored via crystallization. Three examples are shown in Figure 1.5. Sucrose, sugar, is extracted from plants and crystallized to meet a certain particle size distribution, typically in the range of 700–800 μm, to be free of fines, which allows a free-flowing product that does not agglomerate. Finally, the process arrives at purities of >99.5% in an essentially single-step process of a seeded batch crystallization. Table salt also is required to be free flowing and not to agglomerate even in the high relative humidity environment of a kitchen. Here, additives can be employed during the crystallization, which is usually continuously operated evaporation crystallization. Finally, one of the main components of chocolate, cocoa fat has to be crystallized in a certain crystal modification or polymorph to achieve the special mouth taste of chocolate. This modification is unstable at room temperature and achieved via melt crystallization, where the crystals of the desired modification are generated and grown via a temperature program. In addition, additives can be used to stabilize the required modification. The unstable modification of the fatty acid ester can recrystallize to a more stable one, resulting in undesired changes in the appearance of the product.
Figure 1.5 Sugar, table salt, and chocolate as examples of everyday life products, where the properties of the crystalline state determine product properties and where the crystallization is tailored to meet this demand.

In a number of cases, mother liquor is the desired product of the crystallization process. The crystallization of ice from aqueous solutions can be used for freeze concentration of aqueous solutions. One example of everyday life is orange juice that can be freeze concentrated at low temperatures gently and in an energy-efficient way. The concentration of waste from effluent waters is another application.
The application of crystallization in industry ranges from the isolation of the few milligrams of a substance newly synthesized in the laboratory – where a well-defined melting point is used to both achieve and prove a decent purity of the crop and as an identity check – to a mass crystallization carried out in very diverse industries; some products are listed with their annual production volume in Table 1.1.
Table 1.1 Examples for the annual production of crystalline products in various fields.
| Product | Produced in | Production (t/a) |
| Sodium chloride | 2001 in the EU | 38 350 000 |
| Sugar | 2001 in the EU | 15 000 000 |
| Caprolactam | 2002 worldwide | 3 500 000 |
| Ascorbic acid | 2009 worldwide | 110 000 |
| Acetylsalicylic acid | 2008 worldwide | 35 000 |
The equipment used in the industrial crystallization varies widely, from multipurpose batch vessels in the life science industry to highly sophisticated dedicated equipment used for some large volume products.
In the following chapters, the basic concepts of the modern understanding of crystallization will be discussed, such as the internal structure of the crystals and their growth mechanisms or the phase diagrams. Attention will be directed to the purification by crystallization and to ...
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- List of Contributors
- Chapter 1: Crystallization: Introduction
- Chapter 2: Mechanisms of Crystallization
- Chapter 3: Solubility and Solution Equilibria in Crystallization
- Chapter 4: Agglomeration During Crystallization
- Chapter 5: Polymorphism of Crystalline Systems
- Chapter 6: The Influence of Additives and Impurities on Crystallization
- Chapter 7: Purification by Crystallization
- Chapter 8: Characterization of Crystalline Products
- Chapter 9: Basics of Industrial Crystallization from Solution
- Chapter 10: Development of Batch Crystallizations
- Chapter 11: Continuous Crystallization
- Chapter 12: Precipitation
- Chapter 13: Mixing in Crystallization Processes
- Chapter 14: Downstream Processes
- Chapter 15: Melt Crystallization
- Chapter 16: Examples of Realized Continuous Crystallization Processes
- Chapter 17: Design Examples of Melt Crystallization
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Crystallization by Wolfgang Beckmann in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.