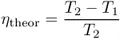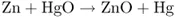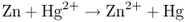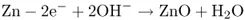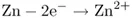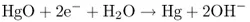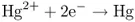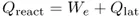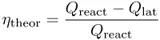![]()
Part I
INTRODUCTION
![]()
Chapter 1
The Working Principles of a Fuel Cell
1.1 Thermodynamic Aspects
1.1.1 Limitations of the Carnot Cycle
Up to the middle of the twentieth century, all human energy needs have been satisfied by natural fuels: coal, oil, natural gas, wood, and a few others. The thermal energy Qreact set free upon combustion (a chemical reaction of oxidation by oxygen) of natural fuels is called the reaction enthalpy or lower heat value (LHV): “lower” because the heat of condensation of water vapor as one of the reaction products is usually disregarded. A large part of this thermal energy serves to produce mechanical energy in heat engines (e.g., steam turbines, various types of internal combustion engines).
According to one of the most important laws of nature, the second law of thermodynamics, the conversion of thermal to mechanical energy Wm is always attended by the loss of a considerable part of the thermal energy. For a heat engine working along a Carnot cycle within the temperature interval defined by an upper limit T2 and a lower limit T1, the highest possible efficiency, ηtheor ≡ Wm/Qreact, is given by
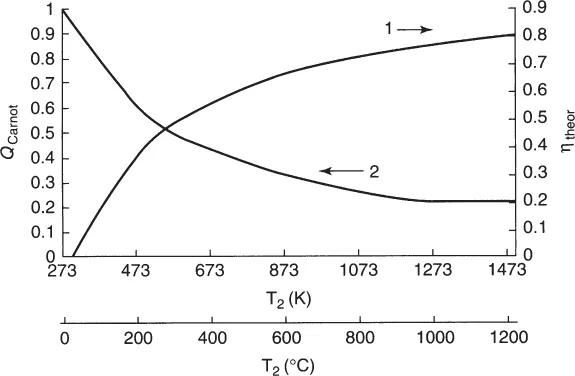
T2 and T1 being the temperatures (in kelvin) of the working fluid entering into and leaving the heat engine, respectively. The Carnot heat QCarnot (or irretrievable heat), for thermodynamic reasons known as the Carnot-cycle limitations is given by QCarnot = (T1/T2)Qreact. There is no way to reduce this loss. For a steam engine operating with superheated steam of 350°C (T2 = 623 K) and release of the exhausted steam into a medium having an ambient temperature of 25° C(T1 = 298K), the maximum efficiency according to equation (1.1) is about 50%, so half of the thermal energy is irretrievably lost. As a matter of fact, the efficiency that can be realized in practice is even lower because of various other types of thermal losses Qloss (e.g., heat transfer out of the engine, friction of moving parts); the total losses (Qexh = QCarnot + Qloss) are even higher. The efficiency ηtheor can be raised by working with a higher value of T2 (Figure 1.1), but losses due to nonideal heat transfer will also increase.
In part, the mechanical energy produced in heat engines is used, in turn, to produce electrical energy in the generators of stationary and mobile power plants. This additional step of converting mechanical into electrical energy involves additional energy losses, but these could be as low as 1 to 2% in a large modern generator. Thus, for a modern thermal power generating plant, a total efficiency ηtotal of about 40% is regarded as a good performance figure.
1.1.2 Electrochemical Energy Conversion
Until about 1850, the only source of electrical energy was the galvanic cell, the prototype of modern storage and throwaway batteries. In such cells, an electric current is produced through a chemical reaction involving an oxidizing agent and a reducing agent, which are sometimes quite expensive. In mercury primary cells, the current is generated through an overall reaction between mercuric oxide (HgO) and metallic zinc (Zn). In the cell, this redox (reducing and oxidizing) reaction occurs via an electrochemical mechanism that is fundamentally different from ordinary chemical mechanisms. In fact, in a reaction following chemical mechanisms, the reducing agent (here, Zn) reacts directly with the oxidizing agent (here, HgO):
the reaction involving a change in the valence states of the metals:
or electron transfer from Zn to Hg (the oxygen simply changing partners). If one were to mix zinc and mercuric oxides as powders in a reaction vessel and cause them to react, the electron transfers between the reacting particles would occur chaotically throughout the space taken up by the reactants, and no electron flow in any particular direction would be observed from the outside. For this reason, all of the chemical energy set free by the reaction would be evolved in the form of heat.
When an electrochemical mechanism is realized, then in the present example, electrons are torn away from the zinc at one electrode by making zinc dissolve in an aqueous medium:
or, essentially,
and are added to mercuric oxide (HgO or Hg2+) at the other electrode, by making the mercury deposit onto the electrode:
or, essentially,
the overall reaction occurring spatially separately at two different electrodes contacting the (aqueous) medium or electrolyte. Reaction (1.3) is zinc oxidation occurring as the anodic reaction at the anode. Reaction (1.4) is mercury reduction occurring as the cathodic reaction at the cathode. These two electrode reactions taken together yield the same products as those in chemical reaction (1.2).
Reactions (1.3) and (1.4) will actually proceed only when the two electrodes are connected outside the cell containing them. Electrons then flow from the zinc anode (the negative pole of the cell) to the mercuric oxide cathode (the positive pole). The cell is said to undergo discharge while producing current. Within the cell, the hydroxyl ions (OH−) produced by reaction (1.4) at the cathode are transferred (migrate) to the anode, where they participate in reaction (1.3). The ions and electrons together yield a closed electrical circuit.
Of the total thermal energy of these two processes, Qreact [the reaction enthalpy ( − ΔH)], a certain part [called the Gibbs reaction energy ( − ΔG)] is set free as electrical energy We (the energy of the current flowing in the external part of the cell circuit). The remaining part of the reaction energy is evolved as heat, called the latent heat of reaction Qlat [or reaction entropy ( − TΔS)] (the latent heat in electrochemical reactions is analogous to the Carnot heat in heat engines):
In summary, in the electrochemical mechanism, a large part of the chemical energy is converted directly into electrical energy without passing through thermal and mechanical energy forms. For this reason, and since the value of Qlat usually (if not always) is small compared to the value of Qreact, the highest possible theoretical efficiency of this conversion mode,
is free of Carnot cycle limitations and may approach unity i.e., 100%).1 Even in this case, of course, different losses Qloss have the effect that the practical efficiency is lower than the theoretical maximum, yet the efficiency will always be higher than that attained with a heat engine. The heat effectively exhausted in the electrochemical mechanism is the sum of the two components mentioned: Qexh = Qlat + Qloss.
Toward the end of the nineteenth century, after the invention of the electric generator in 1864, thermal power plants were built in large numbers, and grid power gradually displaced the galvanic cells and storage batteries that had been used for work in laboratories and even for simple domestic devices. However, in 1894, a German physical chemist, Wilhelm Ostwald, formulated the idea that the electrochemical mechanism be used instead for the combustion (chemical oxidation) of natural types of fuel, such as those used in thermal power plants, since in this case the reaction will bypass the intermediate stage of heat generation. This would be cold combustion, the conversion of chemical energy of a fuel to electrical energy not being subject to Carnot cycle limitations. A device to perform this direct energy conversion was named a fuel cell.
The electrochemical mechanism of cold combustion in fuel cells has analogies in living beings. In fact, the conv...