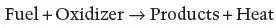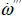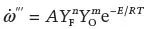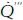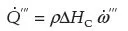![]()
Part 1
Fire Behaviour
![]()
1 An Introduction to Combustion in Organic Materials
JOSE L. TORERO
School of Civil Engineering, The University of Queensland, Brisbane, Queensland, Australia
1.1 Introduction
Combustion is a process by which fuel and oxidizer react to produce a different set of chemical products and heat. The process is intimately linked to the nature of the fuel but also to different transport processes that define the characteristics of the combustion process. This chapter provides a brief and general description of the different processes and of commonly used nomenclature.
Discussing combustion of organic materials needs to start with simple definitions that enable the description of the main phenomena involved. Organic materials can be defined as carbon-based materials, which can be divided into natural materials and processed materials. Processed materials are those generated through some modification that alters the physical or chemical characteristics of the natural materials. Natural materials include vegetation, decomposed vegetation, coal or the large group known as hydrocarbons (oils, tars, etc.). Processed materials include timber, plastics, petrol and many other industrial products. It is important to emphasize that many inorganic materials will also combust, but these will not be discussed here.
From the combustion perspective natural or processed organic materials are no different; in both cases the combustion process can be described as a chemical reaction that is defined by the following generic expression:
The ‘fuel’ being the organic material and the ‘oxidizer’ being oxygen extracted in most cases from air. Chemical reactions associated with combustion are exothermic, thus the products are released with a significant amount of energy. The specific energy (i.e. joules produced per kilogram of the material) tends to be extremely high when combustion processes are compared to other energy-generating mechanisms (electric batteries, fuel cells, etc.; Fernandez-Pello, 2002). Thus, combustion of organic fuels has been a preferred source of energy.
Combustion processes have been controlled for at least 100 000 years (Stahl, 1984; James, 1989) and humans have learnt to harness the energetic content of organic fuels for cooking, comfort and power. Until the industrial revolution combustion was poorly understood and was limited to controlled burning of natural fuels (i.e. coal, wood). The Industrial Revolution generated the first true understanding of combustion (Faraday, 1908), the massive use of organic fuels, and the first attempts to modify organic compounds to produce more efficient fuels (Frank, 2005). Today, combustion of modified organic fuels represents more than 85% of the total worldwide energy production (Jacobson, 2009).
A different form of combustion is known as fire. Fire is the uncontrolled chemical oxidation of organic fuels that is generally associated with destruction. In fire, the heat of the combustion process serves to sustain the uncontrolled burning of any adjacent organic fuels. Fires occur in many forms and scales and are generally deemed as detrimental to humans, economies and the environment. Natural fires include peat, forest and underground fires (Rein, 2009) while infrastructure fires affect buildings of all natures and sizes (Torero and Rein, 2009).
To be able to discuss controlled or uncontrolled combustion of organic solids it is important to understand the fundamental underlying physical and chemical phenomena involved. The following sections will therefore present a brief discussion of combustion related processes.
1.2 The Reactive Zone
Given that combustion is an exothermic chemical reaction, it has to happen in a specific location. The location is given by the way reactants are delivered and by the local temperature. If the temperature is too low or in the absence of fuel or oxidizer combustion cannot occur, thus there are specific conditions that will sustain combustion. If adequate conditions are attained at a specific location the chemical reaction will proceed at a defined rate (
; 1/seconds). The dot denotes per unit time while the triple prime per unit volume. The rate is a function of the supply of reactants and the temperature as indicated by
Equation 1.2:
Equation 1.2 is a common way to represent typical combustion reactions and is one potential form of the family of Arrhenius type equations that describe the reaction rate on the basis of kinetic theory. This expression indicates that the presence of oxygen and fuel is necessary with them appearing through their respective concentrations (YF is the concentration of fuel while YO is the concentration of oxygen). Both terms are dimensionless. The coefficients n and m are called the reaction orders and denote the sensitivity of the rate to each of the reactants. The exponential term brings the dependency on temperature. This term shows that the reaction rate will be very small unless the temperature reaches a threshold that makes the product RT comparable to the activation energy (E). The constant R is the gas constant (R = 8.314 J/mol K) and T is the temperature in kelvin. The product RT represents the energy accumulated in the molecules as the temperature increases; thus when the temperature reaches a certain threshold the number of collisions induced by the increase in kinetic energy results in the breakdown of the molecules and the onset of the reaction. The rate will then continue to increase as the temperature increases. The term A (1/second) is just the constant of proportionality that links the reaction rate to the parameters controlling it.
The magnitude of the activation energy (E) will define the sensitivity of the reaction rate to temperature: the larger the value of E (high activation energy) the more sensitive the reaction rate is to variations of the reactants’ temperature. Combustion reactions have very high activation energies, thus are extremely sensitive to temperature.
As the molecules of reactants combust to produce products they release energy. The energy produced per unit time and volume of reactants is denoted by
(joules/m
3.s) and is given by
Equation 1.3:
where ∆ HC is the heat of combustion or energy produced per kilogram of fuel (joules/kgFUEL) while ρ is the global density of the reactants. The heat of combustion depends on the organic fuel, and typical values are tabulated in most combustion books (Glassman and Yetter, 2008). Table 1.1 lists the heat of combustion of a small group of organic materials.
Table 1.1 Representative heats of combustion (ΔHC) of different organic materials.
| Fuel | ∆ HC (MJ/kgFUEL) |
| Hydrogen | 141.80 |
| Propane | 50.35 |
| Gasoline | 47.30 |
| Paraffin | 46.00 |
| Kerosene | 46.20 |
| Coal (lignite) | 15.00 |
| Wood | 15.00 |
| Peat (dry) | 15.00 |
| PVC (Polyvinyl chloride) | 17.50 |
| PE (Polyethylene) | 44.60 |
The energy produced is then transferred back to the products or lost to the environment. The fraction of the energy transferred to the products will define the temperature of the products and is normally referred to as the flame temperature, TF. If the flame temperature is high enough then heat will be transferred to fresh reactants leading to a self-sustained reaction; if the temperature is too low then the reaction will have to be assisted by an external supply of energy.
Equation 1.2 shows clearly the requirements for a sustained reaction: fuel and oxidizer have to be delivered in sufficient quantities to maintain adequate concentrations, and the flame temperature has to be high enough to guarantee sufficient heat transfer to the reactants to achieve a high enough temperature that will result in a strong reaction rate. Thus, ignition requires the supply of reactants within the necessary concentrations (flammability limits) and a supply of energy to initiate the reaction and increase the reaction rate to a level where it becomes self-sustained (critical ignition energy). Extinction can be then achieved by precluding the arrival of either reactant or by enhancing heat losses so that the temperature of the flame decreases below the threshold that will enable sufficient energy production to self-sustain the reaction.
Combustion chemistry has been briefly discussed here using a simple approach; nevertheless, the complexity of this process can be significant, involving many reactions and different transport processes (heat and mass). For a more detailed discussion the reader is directed to Glassman and Yetter (2008).
While combustion is a chemical reaction and thus the reaction rates are expressed in chemical terms, it is in many cases a process controlled by the transport of heat and mass. How fast the reactants reach the reaction zone determines the production of energy, and how fast the energy is transferred towards the reactants will determine the vigour of the reaction rate.
The simplest way to separate different forms of combustion is by specifying the mechanisms by which reactants reach the reaction zone. The first case is the premixed flame; in this case fuel and oxidizer are mixed in concentrations that are adequate for combustion to occur. Here, no mass transport is necessary and the reaction consumes the reactants as fast as the energy is transferred towards the unburnt gases. Thus the problem is dominated purely by heat transfer. The other extreme is the non-premixed flame; in this case fuel and oxidizer are separate and the energy produced is determined only by the rate at which reactants can be delivered to the flame. In this case the process is dominated purely by mass transfer, so many texts refer to non-premixed combustion as ‘diffusion flames’. Premixed flames will require fuel and oxidizer to both be in the gas phase, and they are thus commonly used for burners and other controlled energy-generating processes such as engines or turbines. Non-premixed flames are typical of condensed-phase fuels (liquids or solids) where the fuel is supplied at a rate that is defined by the amount of energy delivered by the combustion reaction. This energy is what enables the phase of the fuel to change. Non-premixed combustion is typical in uncontrolled processes such as fires but can also be used in b...