
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Earth Materials
About this book
Minerals and rocks form the foundation of geologic studies. This new textbook has been written to address the needs of students at the increasing number of universities that have compressed separate mineralogy and petrology courses into a one- or two-semester Earth materials course.
Key features of this book include:
- equal coverage of mineralogy, sedimentary petrology, igneous petrology and metamorphic petrology;
- copious field examples and regional relationships with graphics that illustrate the concepts discussed;
- numerous case studies to show the uses of earth materials as resources and their fundamental role in our lives and the global economy, and their relation to natural and human-induced hazards;
- the integration of earth materials into a cohesive process-based earth systems framework;
- two color thoughout with 48 pages of four color.
Readership: students taking an earth materials, or combined mineralogy and petrology course in an earth science degree program. It will also be useful for environmental scientists, engineering geologists, and physical geographers who need to learn about minerals, rocks, soil and water in a comprehensive framework.
A companion website for this book is available at: www.wiley.com/go/hefferan/earthmaterials.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Earth materials and the geosphere
1.1 EARTH MATERIALS
- 1 Minerals are solid, so they do not include liquids and gases. Minerals are solid because all the atoms in them are held together in fixed positions by forces called chemical bonds (Chapter 2).
- 2 Minerals are naturally occurring. This definition excludes synthetic solids produced through technology. Many solid Earth materials are produced by both natural and synthetic processes. Natural and synthetic diamonds are a good example. Another example is the solid materials synthesized in high temperature and high pressure laboratory experiments that are thought to be analogous to real minerals that occur only in the deep interior of Earth.
- 3 Minerals usually form by inorganic processes. Some solid Earth materials form by both inorganic and organic processes. For example, the mineral calcite (CaCO3) forms by inorganic processes (stalactites and other cavestones) and is also precipitated as shell material by organisms such as clams, snails and corals.
- 4 Each mineral species has a specific chemical composition which can be expressed by a chemical formula. An example is common table salt or halite which is composed of sodium and chlorine atoms in a 1 : 1 ratio (NaCl). Chemical compositions may vary within well-defined limits because minerals incorporate impurities, have atoms missing, or otherwise vary from their ideal compositions. In addition some types of atoms may substitute freely for one another when a mineral forms, generating a well-defined range of chemical compositions. For example, magnesium (Mg) and iron (Fe) may substitute freely for one another in the mineral olivine whose composition is expressed as (Mg,Fe)2SiO4. The parentheses are used to indicate the variable amounts of Mg and Fe that may substitute for each other in olivine group minerals (Chapter 3).
- 5 Every mineral species possesses a long-range, geometric arrangement of constituent atoms or ions. This implies that the atoms in minerals are not randomly arranged. Instead minerals crystallize in geometric patterns so that the same pattern is repeated throughout the mineral. In this sense, minerals are like three-dimensional wall paper. A basic pattern of atoms, a motif, is repeated systematically to produce the entire geometric design. This long-range pattern of atoms characteristic of each mineral species is called its crystal structure. All materials that possess geometric crystal structures are crystalline materials. Solid materials that lack a long-range crystal structure are amorphous materials, where amorphous means without form; without a long-range geometric order.
1.2 THE GEOSPHERE
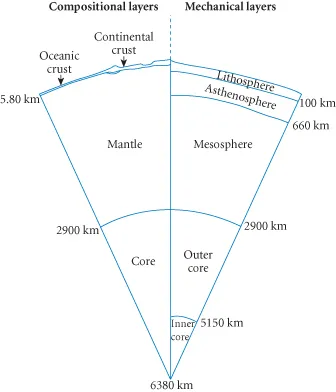
1.2.1 Compositional layers
1.2.2 Mechanical layers
Table of contents
- Cover
- Table of Contents
- Dedication
- Title
- Copyright
- Preface
- Acknowledgments
- Chapter 1: Earth materials and the geosphere
- Chapter 2: Atoms, elements, bonds and coordination polyhedra
- Chapter 3: Atomic substitution, phase diagrams and isotopes
- Chapter 4: Crystallography
- Chapter 5: Mineral properties and rock-forming minerals
- Chapter 6: Optical identification of minerals
- Chapter 7: Classification of igneous rocks
- Chapter 8: Magma and intrusive structures
- Chapter 9: Volcanic features and landforms
- Chapter 10: Igneous rock associations
- Chapter 11: The sedimentary cycle: erosion, transportation, deposition and sedimentary structures
- Chapter 12: Weathering, sediment production and soils
- Chapter 13: Detrital sediments and sedimentary rocks
- Chapter 14: Biochemical sedimentary rocks
- Chapter 15: Metamorphism
- Chapter 16: Metamorphism: stress, deformation and structures
- Chapter 17: Texture and classification of metamorphic rocks
- Chapter 18: Metamorphic zones, facies and facies series
- Chapter 19: Mineral resources and hazards
- References
- Index
- Periodic table of the elements
- Table of chemical elements
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app