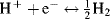![]()
Chapter 1
Hydrogen Reactions on Nanostructured Surfaces
Holger Wolfschmidt and Odysseas Paschos
Department of Physics, Technische Universität München, Garching Germany
Ulrich Stimming
Department of Physics, Technische Universität München and ZAE Bayern Division 1, Garching Germany
Hydrogen catalysis is an important scientific field since hydrogen reactions (e.g., hydrogen evolution and hydrogen oxidation) play a key role in electrochemical devices such as fuel cells and electrolyzers. The latter devices have the potential to provide clean and sustainable energy with high efficiencies. This chapter reviews hydrogen catalysis in detail. Details on hydrogen reaction studies from theoretical and experimental perspectives are presented. The former usually complement the results from experimental studies and are used to strengthen them. Various systems that have been explored throughout the years are reviewed. These include model surfaces as well as applied systems. Model catalyst systems comprise Pt and Pd nanoislands deposited on planar surfaces of inert supports, high-quality single-crystal materials, or single nanoparticles created with scanning tunneling microscopy tips. Applied systems consist of metallic nanoparticles deposited on high-surface-area carbon supports. Theory versus experiment, and model versus applied systems are reviewed in detail, and useful insights for hydrogen reactions in these systems are demonstrated
1.1 Introduction
Whereas the nineteenth century was the stage of the steam engine and the twentieth century was the stage of the internal-combustion engine, it is likely that the twenty-first century will be the stage of the fuel cell. Fuel cells have captured the interest of people around the world as one of the next great energy alternative. They are now on the verge of being introduced commercially, revolutionizing the present method of power production. Fuel cells can use hydrogen as fuel and oxygen or air as oxidant, offering the prospect of supplying the world with clean, sustainable electrical power, heat, and water.
This chapter focuses on hydrogen reactions such as the hydrogen oxidation reaction (HOR) and the hydrogen evolution reaction (HER). These reactions are of utmost importance in developing and improving fuel cell devices. The discussion here is directed principally toward hydrogen electrocatalysis from an experimental as well as theoretical perspective. Starting with an overview on the fundamentals of hydrogen reactions in Section 1.2, studies on single crystals as well-defined and high-quality surfaces are reviewed. An introduction to theoretical work calculating important fundamentals for hydrogen catalysis regarding material properties is then discussed. As predicted by theory, the behavior of nanostructured and bimetallic surfaces differs from that of bulk material. Similar findings supporting the theoretical predictions are shown for large nanostructured surfaces as well as single particles. The section concludes with a short overview of carbon-based catalysts.
The fundamentals of hydrogen reactions are reviewed in Section 1.2. Starting from the general reversible hydrogen reaction, the different reaction pathways suggested by Volmer, Heyrowsky, and Tafel are introduced. Because of the importance of the hydrogen adsorption mechanism and the important findings with new experimental techniques, a short overview of results obtained since the late 1990s is given. An introduction to the correlation between catalytic behavior and the catalyst material significance of this correlation, completes this section using experimental and theoretical calculations, with a conclusion regarding the long-range.
Single crystals and well-defined surfaces play a very important role in surface science. Many scientific contributions are available that study these well-defined surfaces. Section 1.3 introduces the electrochemical behavior toward hydrogen reactions on Pt, Au, and Pd surfaces. The quality of single crystals rapidly increased in the 1990s, resulting in new and different insights. Because of the importance of Pt as a catalyst, the main part of this section focuses on this element. The dependence of the crystallographic orientation toward adsorption as well as electrocatalytic activity is discussed. An introduction to Pd as a catalyst material with the property to absorb hydrogen and Au as an inert support material is the last topic in that section.
Besides experimental work, numerous theoretical calculations for hydrogen catalysis have been performed. Computational methods such as density functional theory (DFT) and Monte Carlo simulations are powerful tools in surface science and catalysis. Theoretical as well as experimental work has been combined in several scientific publications and complement each other well. The first principles of theoretical techniques and theoretical results are shown in Section 1.4. As a main topic, the adsorption behavior of hydrogen is considered and the d-band model is introduced. Calculations regarding the hydrogen oxidation reaction and the influence of different reactions pathways are also shown. Theoretical calculations of metals on thin films and supported on various foreign metals are reviewed and are correlated with experimental findings.
The chemical behavior of metal nanoparticles often differs from that of bulk metal. Different effects such as particle size, interparticle distance, and support effects have to be considered in this nanometer-scale regime. Since Pd and Pt are important materials in catalysis, much work was done in the last few decades describing the abovementioned effects. In particular, multilayers, monolayers, and submonolayers of Pd and Pt onto foreign metal supports have shown unexpected behavior. Pd on Au(111) regarding several electrochemical properties introduces this section. Different types of adsorption, absorption, and desorption behavior as well as electrocatalytic activity toward hydrogen reactions are shown and discussed. The deposition of Pd on other supports and the influence of hydrogen reactions hindered by adsorbing foreign adsorbates as well as investigations of Pt overlayers on Au(111) are also discussed. A summary and detailed discussion in Section 1.5 also includes theoretical aspects.
As mentioned above, planar surfaces are thoroughly investigated and serve as widely accepted reference systems with high-quality, reproducible results. For local investigation of small structures, new approaches and setups have to be designed and applied. For this purpose, the electrochemical scanning tunneling microscope (EC-STM) has been modified by several groups in order to create small nanoparticles and nanoparticle arrays and also to investigate corrosion, deposition, dissolution, and reactivity. Due to the tunneling effect, high resolution is achievable and thus leads to a precise technique with atomic resolution. The STM tip can be used in different ways in the electrochemical environment to create and investigate local reactivity of nanostructures. Experimental and theoretical results are compared and are shown to complement each other. Specifically, the activity of a single Pd particle is shown. A discussion of the experimental results of the stability of Pd particles deposited on Au(111) and their reactivity toward HER follows. A summary completes Section 1.6 with a comparison between results obtained at extended Pd nanostructured Au(111) surfaces and single Pd particles.
Section 1.7 presents an overview of studies performed on carbon-based systems. Since carbon has high electrical conductivity, is relatively inexpensive to use, and is highly available, it has been the favored support material for many years. Of the many scientific contributions, only a few can be presented here regarding the mechanism of HER and HOR using metallic nanoparticles with carbon-based supports. The reactivity of these catalysts for hydrogen reactions and CO oxidation is also of major interest. These catalyst systems include glassy carbon, carbon nanofibers, Vulcan, and carbon black for support for metallic nanoparticles, and the more highly oriented and defined pyrolytic graphite (HOPG) are also presented and discussed.
1.2 Fundamentals of Hydrogen Reactions
1.2.1 Hydrogen Catalysis
Over the years a number of studies have been performed in order to investigate the characteristics of hydrogen-related reactions. The general reversible reaction is as follows:
Its standard potential is set to 0 V. In the case of proton discharge to form molecular hydrogen the reaction is called a hydrogen evolution reaction (HER), while the reverse pathway describes the hydrogen oxidation reaction (HOR). However, for the...